- EN - English
- CN - 中文
Isolation of In Vitro Osteoblastic-Derived Matrix Vesicles by Ultracentrifugation and Cell-Free Mineralization Assay
体外成骨细胞来源基质囊泡的超速离心分离及无细胞成矿分析方法
发布: 2025年04月05日第15卷第7期 DOI: 10.21769/BioProtoc.5258 浏览次数: 1379
评审: Wendy Leanne HempstockChiara AmbrogioAnonymous reviewer(s)

相关实验方案

外周血中细胞外囊泡的分离与分析方法:红细胞、内皮细胞及血小板来源的细胞外囊泡
Bhawani Yasassri Alvitigala [...] Lallindra Viranjan Gooneratne
2025年11月05日 1448 阅读
Abstract
Matrix vesicles (MVs) represent a heterogeneous group of spherical membrane-bound extracellular vesicles in the range of 100–200 nm in diameter secreted by mineralizing osteoblasts. The initial synthesis of the amorphous calcium phosphate occurs within the confines of the intracellular MVs, which are capable of transporting Pi and Ca2+ into the MV lumen. Thus, understanding the initial process of MV-mediated mineralization is critical in developing better therapeutic strategies for various bone-related disorders such as osteoporosis and addressing ectopic calcification of soft tissues. Although various techniques and commercially available kits are now available for isolating MVs, isolating a pure population of MVs is challenging mainly because of their variable size and lack of consensus protein markers. This ultracentrifugation-based protocol ensures high purity of isolated MVs by removing other contaminated extracellular vesicles and cellular debris through sequential centrifugation steps but also allows downstream functional mineralization assays of the isolated MVs.
Key features
• Simple and rapid high-quality isolation of MVs from in vitro culture of mineralizing osteoblasts by ultracentrifugation.
• Use of isolated MVs for various functional assays such as mineralization efficacy.
• Cell-free mineralization assay to determine intrinsic mineralization efficacy of the isolated MVs under desired experimental conditions.
Keywords: Extracellular vesicles (细胞外囊泡)Graphical overview
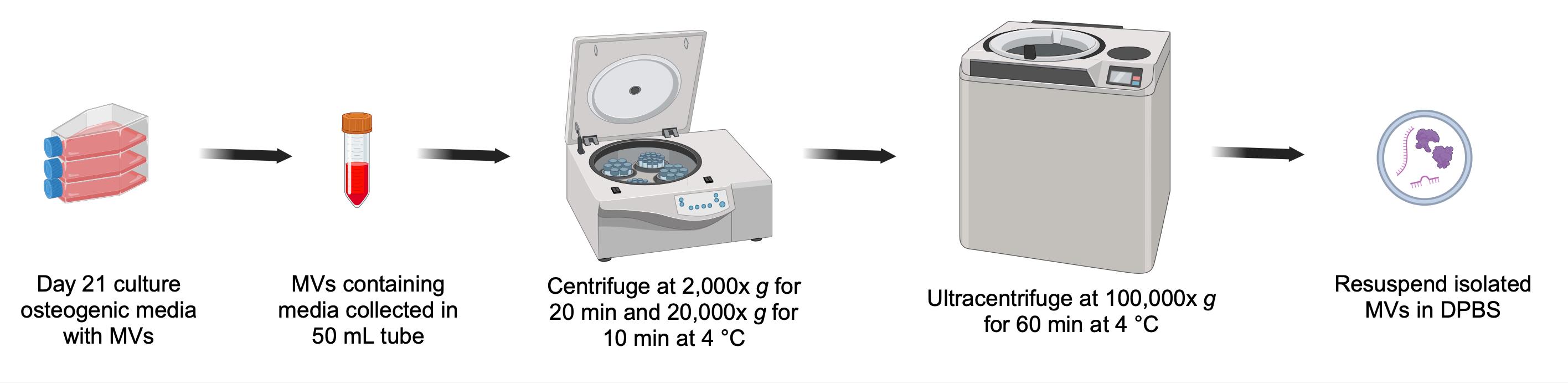
Overall scheme for isolation of matrix vesicles (MVs) from osteogenic media
Background
Matrix vesicles (MVs) are extracellular membrane-bound nanovesicles enriched with various enzymes that transport proteins to facilitate the synthesis of hydroxyapatite (HA) and transmembrane flux of Ca2+ and PO43- to their lumen. The initial amorphous HA forms on the inner leaflet of MVs and acts as the primary nucleation center of mature HA crystals, which continue to grow to form calcified nodules [1,2].
Based on recent studies, MVs are not only involved in mineralization but also carry nucleic acid and protein cargo within their lumen that participates in autocrine and paracrine exchange of intracellular or intercellular signals [3,4]. Because of this functional role of MVs in signal transduction and the initial nucleation centers for bone formation, there is an urgent need for a better understanding of the functional physiology of MVs and how their functions are affected by various inflammatory conditions such as chronic inflammation and osteoporosis [5].
As more researchers continue to decipher and acknowledge various physiological roles of MVs, many different methods of MVs isolation have been developed, each having its own pros and cons as well as differences in purity and required isolation time [6,7]. Some of the most widely used methods include ultracentrifugation, precipitation kits, size exclusion chromatography, ultrafiltration, and magnetic bead technologies [8]. Choosing a suitable isolation method for MVs depends on the volume and type of starting material, the scientific query to be addressed, and subsequent analysis of the isolated MVs. Moreover, the protein and nucleic acid composition of MVs is influenced by the method of isolation protocol used. Hence, it is crucial to determine the most appropriate isolation protocol in order to ensure accurate interpretation and data reproducibility.
Here, we describe the most commonly used ultracentrifugation-based protocol for MV isolation, which we recently used to unravel the role of NCX3 in facilitating Ca2+ entry within the MVs during the initial mineralization process [9]. This method of MV isolation offers the advantage of isolating native, unmodified MVs from culture media with relative ease [10]. The isolated MVs exhibit high purity and retain their intrinsic mineralization ability, features that can be leveraged to investigate the mineralizing properties of isolated MVs under desired experimental conditions in a cell-free environment. The potential drawbacks of the method include the relatively large scale of osteoblast culture, required access to an ultracentrifuge, and possible co-isolation of contaminants as this method is based on different sedimentation rates of particles that differ in size and density.
Materials and reagents
Biological materials
1. MC3T3-E1 subclone 4 cell line, obtained from American Type Culture Collection (Manassas, VA; ATCC #CRL-2593). Cells should be grown to 70%–80% confluency in growth media (see Recipe 3) and maintained in a humidified environment at 37 °C in the presence of 5% CO2 in a cell culture incubator
Reagents
1. MEM Alpha (Gibco, catalog number: A10490-01)
2. Ascorbic acid (Sigma-Aldrich, catalog number: A4403)
3. Exosome-depleted FBS (Gibco, catalog number: A2720801)
4. Penicillin-streptomycin (100×) (Invitrogen, catalog number: 15140122)
5. Trypsin-EDTA (0.25%), phenol red (Gibco, catalog number: 25200072)
6. Dulbecco’s phosphate-buffered saline (DPBS) (Gibco, catalog number: 14190144)
7. Sodium phosphate dibasic (Na2HPO4) (Sigma, catalog number: S9763)
8. Pierce BCA Protein Assay kit (Thermo Scientific, catalog number: 23227)
9. Radioimmunoprecipitation assay (RIPA) lysis buffer (EMD Millipore, catalog number: 20-188)
10. Protease inhibitor cocktail (Sigma, catalog number: P8340)
11. HCl 37% (Acros Organics, catalog number: 124630010)
12. Acetic acid, glacial (Sigma, catalog number: 695092)
13. Calcium colorimetric assay (Sigma, catalog number: MAK022-1KT)
14. Bovine serum albumin (BSA) (Thermo Scientific Chemicals, catalog number: 9048-46-8)
15. Sterile water (Fisher Scientific, catalog number: SH3119101)
Solutions
1. Ascorbic acid solution (see Recipes)
2. Na2HPO4 solution (see Recipes)
3. Growth media (see Recipes)
4. Osteogenic media (see Recipes)
5. 0.6 N HCl (see Recipes)
Recipes
1. Ascorbic acid solution (1,000× stock)
| Reagent | Final concentration | Weight/Volume |
|---|---|---|
| Ascorbic acid | 50 mg/mL | 50 mg |
| Sterile water | 1 mL |
Store at -20 °C protected from light for up to 2–4 weeks.
2. Na2HPO4 solution (1 M)
| Reagent | Final concentration | Weight/Volume |
|---|---|---|
| Na2HPO4 | 1 M | 4.26 g |
| Sterile water | 30 mL |
Store at room temperature for up to 2–3 months.
3. Growth media
| Reagent | Final concentration | Volume |
|---|---|---|
| MEM Alpha | 1× | 44.5 mL |
| FBS | 10% v/v | 5 mL |
| Penicillin-streptomycin (100×) | 500 μL | |
| Total | 50 mL |
Store at 4 °C for up to 2 weeks. FBS was heat inactivated at 55 °C for 30 min prior to use.
4. Osteogenic media
| Reagent | Final concentration | Volume |
|---|---|---|
| MEM Alpha | 1× | 44.35 mL |
| FBS | 10% v/v | 5 mL |
| Penicillin-streptomycin (100×) | 1× | 500 μL |
| Ascorbic acid (Recipe 1) | 50 μg/mL | 50 μL |
| Na2HPO4 (Recipe 2) | 3 mM | 150 μL |
| Total | 50 mL |
Always prepare fresh and protect from light.
5. 0.6 N HCl
a. Measure 4.97 mL of 37% HCl using a 5 mL pipette in a 100 mL beaker.
b. Add distilled water until the volume reaches 100 mL.
c. Mix thoroughly with a magnetic stirring bar inside the beaker.
Laboratory supplies
1. 1.5 mL microcentrifuge tubes (USA Scientific, catalog number: 1415-2500)
2. 15 mL conical tubes (Sarstedt, catalog number: 62.553.205)
3. 50 mL conical tubes (Sarstedt, catalog number: 62.547.205)
4. Serological pipettes 10 mL (Sarstedt, catalog number: 86.1254.001)
5. Serological pipettes 25 mL (Sarstedt, catalog number: 86.1685.001)
6. Tissue culture flask, 150 cm2, canted neck, vented cap (Corning, catalog number: 430825)
7. Tissue culture flask, 25 cm2 filter (Greiner, catalog number: 690175)
8. 0.45 μm sterile syringe filters (Sarstedt, catalog number: 83.1826)
9. Open-top thin-wall ultracentrifuge tube, 38.5 mL capacity (Beckman Coulter, catalog number: 344058)
10. TEM Grids G300 (SPI Supplies, catalog number: 2030C-XA) for electron microscopy
11. 22 mm collagen-coated glass coverslip (Sigma, catalog number: CLS354089)
12. Forceps (Sigma, catalog number: F4017)
13. 100 mL beaker (Fisher Scientific, catalog number: FB100100)
14. Magnetic stirring bar (Fisher Scientific, catalog number: 14-512-126)
15. 12-well cell culture plates (Fisher Scientific, catalog number: 07-200-82)
16. Large-orifice pipette tips (Fisher Scientific, catalog number: 02-707-134)
Equipment
1. CO2 cell culture incubator (e.g., Thermo Scientific, catalog number: 51033557)
2. Ultra-centrifuge with a swinging bucket rotor [e.g., Beckman Coulter, model: Optima LE-80K with SW32 Ti swinging-bucket rotor (Beckman Coulter, catalog number: 369694)]
3. Electron microscope (Hitachi, model: HF5000) equipped with an adapter for X-ray microanalysis, energy dispersive spectrometer (EDS, Oxford Instruments)
4. Nanosight LM10-HS instrument (Malvern Instruments, Worcestershire, UK), equipped with an sCMOS camera and 638 nm laser
Software and datasets
1. GraphPad Prism version 9.5.1 (GraphPad Software, San Diego, CA, USA)
2. BioRender (https://BioRender.com, Toronto, ON, Canada)
Procedure
文章信息
稿件历史记录
提交日期: Oct 4, 2024
接收日期: Feb 25, 2025
在线发布日期: Mar 14, 2025
出版日期: Apr 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Sheikh, I. A., Kiela, P. R. and Ghishan, F. K. (2025). Isolation of In Vitro Osteoblastic-Derived Matrix Vesicles by Ultracentrifugation and Cell-Free Mineralization Assay. Bio-protocol 15(7): e5258. DOI: 10.21769/BioProtoc.5258.
分类
细胞生物学 > 细胞器分离 > 胞外囊泡
细胞生物学 > 细胞成像 > 电子显微镜
细胞生物学 > 细胞运动 > 细胞运动性
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










