- EN - English
- CN - 中文
A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies
基于参与者来源的异种移植小鼠模型:解析HIV自体控制机制并评估免疫疗法
(*Contributed equally to this work, §Co-senior authors) 发布: 2025年04月05日第15卷第7期 DOI: 10.21769/BioProtoc.5254 浏览次数: 2486
评审: Marco Di GioiaDavide Botta
Abstract
Human immunodeficiency virus (HIV) remains a global health challenge with major research efforts being directed toward the unmet needs for a vaccine and a safe and scalable cure. Antiretroviral therapy (ART) suppresses viral replication but does not cure infection and so requires lifelong adherence. HIV-specific CD8+ T-cell responses play a crucial role in long-term HIV control as demonstrated in elite controllers, highlighting their potential in HIV cure strategies. Various HIV mouse models—including the human-hematopoietic stem cell (Hu-HSC) mouse, the bone marrow, liver, and thymus (BLT) mouse, and the human peripheral blood leukocyte (Hu-PBL) mouse—have deepened the understanding of HIV dynamics and facilitated the development of therapeutics. We developed the HIV participant-derived xenograft (HIV PDX) mouse model to enable long-term in vivo evaluation of bona fide autologous T-cell mechanisms of HIV control, including the antiviral activity of primary memory CD8+ (mCD8+) T cells taken directly from people with or without HIV, as well as testing potential immunotherapies. Additionally, this model faithfully recapitulates virus escape mutations in response to sustained CD8+ T-cell pressure, enabling the assessment of strategies to curb virus escape. In this model, NSG mice are engrafted with purified memory CD4+ (mCD4+) cells and infected with HIV; then, they receive autologous CD8+ T cells or T-cell products. Key advantages of this model include the minimization of graft-versus-host disease (GvHD), which severely limits peripheral blood mononuclear cell (PBMC) or total CD4-engrafted mice, the ability to evaluate long-term natural donor-specific T-cell responses in vivo, and the lack of use of human fetal tissues required for most humanized mouse models of HIV.
Key features
• Long-term evaluation of bona fide autologous T cells.
• Evaluation of immunomodulating drugs and T-cell products.
• The protocol requires access to a BSL2+ tissue culture room, BSL2+ animal facility, and 6+ weeks to complete.
Keywords: HIV (HIV)Graphical overview
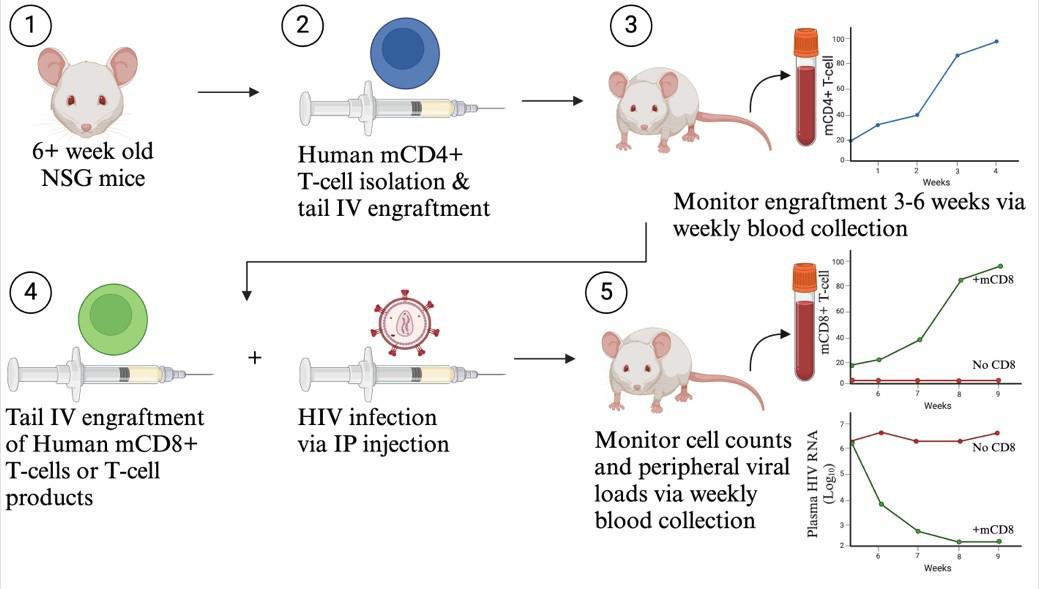
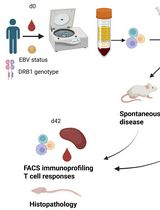
HIV participant-derived xenograft (HIV PDX) mouse model. (1) Use >6-week-old NSG mice. (2) Purify human memory CD4+ T cells and engraft via tail intravenous (IV) injection. (3) Monitor mCD4+ T-cell counts for 3–6 weeks until levels exceed 50 mCD4+ T cells/µL. (4) Infect with HIV via intraperitoneal (IP) injection and engraft autologous CD8+ T cells or T-cell products via tail IV injection. (5) Monitor cell counts and plasma viral loads via weekly blood collection.
Background
Human immunodeficiency virus (HIV) remains a global health challenge with 39.9 million people with HIV (PWH) globally and 1.3 million new infections occurring annually [1]. HIV replication can be suppressed by antiretroviral therapy (ART), allowing PWH to maintain undetectable viral levels. ART prevents both disease progression and viral transmission to sexual partners; however, ART does not cure the infection and thus requires lifelong adherence [2,3]. Major research efforts are being directed toward the unmet needs for a vaccine and a safe and scalable cure. In a minor subset of PWH, termed elite controllers, the HIV-specific CD8 T-cell responses play a crucial role in the spontaneous control of viral replication in the absence of ART [4]. Elucidating the mechanisms of HIV control demonstrated by elite controllers may lead to vaccination or immunotherapeutic strategies to engender immune control in the general population of PWH. A mouse model of HIV that recapitulates key features (as well as limitations, such as immune escape) of CD8+ T cell–mediated control would greatly facilitate these efforts.
Murine cells do not support HIV infection or replication. This has led to the development of “humanized mice” where immunodeficient mouse strains are xenografted with human cells and/or tissues to generate CD4+ T cells supporting HIV replication [5–7]. Notable models include i) the human peripheral blood leukocyte (Hu-PBL-SCID) model, generated by injection of human PBMCs into the peritoneal cavity of CB17-severe immunodeficiency (SCID) mice [8], ii) the human hematopoietic stem cell (Hu-HSC) model, generated by sublethal irradiation of newborn NOD SCID IL2rnull (NSG) mice and reconstitution with human hematopoietic stem cells (HSCs) derived from fetal liver, cord blood, or bone marrow [9,10], and iii) the bone marrow, liver, and thymus (BLT) model, generated by implantation of NSG mice with fetal liver and thymus tissue and then sublethally irradiated and injected with Hu-HSCs [11,12]. These HIV mouse models have deepened the understanding of HIV dynamics and facilitated the development of therapeutics. However, the need and ethical considerations related to the use of human fetal tissues in addition to the onset of graft-versus-host disease (GvHD) limit these mouse models [7,13].
We developed the HIV participant-derived xenograft (HIV PDX) mouse model to enable long-term in vivo evaluation of bona fide autologous T-cell mechanisms of HIV control, including the antiviral activity of primary memory CD8+ (mCD8+) T cells taken directly from adult PWH or HIV-negative donors. One major advantage of this model is the minimization of GvHD, allowing for experimental timelines greater than one year. This was achieved by excluding naive T cells based on the rationale that these, rather than memory T cells, give rise to GvHD. This model robustly recapitulates virus escape mutations in response to sustained CD8 T-cell pressure, enabling the assessment of strategies to curb virus escape. This model uniquely offers the opportunity to test autologous T cells, engineered T-cell therapies, immunomodulatory agents, and combinations thereof. Specifically, in the HIV PDX model, NSG mice are engrafted intravenously with mCD4+ T cells. Following mCD4+ T-cell expansion, mice are infected with HIV and further receive autologous mCD8+ T cells or T-cell products. If desired, immunomodulating agents or ART can be administered. We have leveraged this model to provide pre-clinical efficacy data on T-cell therapy products prior to initiating a clinical trial (NCT04975698) and to assess a targeted delivery platform for IL-15 [14]. The HIV PDX model thus provides a valuable platform in which to examine the roles of CD8+ T cells in natural HIV control as well as to bridge the development of T cell–focused immunotherapeutics from in vitro studies to clinical HIV trials [14].
Materials and reagents
Biological materials
1. NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ mice (The Jackson Laboratory, strain: 005557, 6–8 weeks of age), hereafter referred to as NSG mice
2. Human embryonic kidney 293T (HEK293T) cells (American Type Culture Collection, catalog number: CRL-3216)
3. TZM-bl cells (JC53bl-13) (NIH AIDS Research and Reference Reagent Program, catalog number: ARP-8129)
4. HIV JR-CSF plasmid (NIH AIDS Research and Reference Reagent Program, catalog number: ARP-2708)
5. Human PBMCs (see ethical considerations)
Reagents
1. DMEM (Gibco, catalog number: 11965084)
2. RPMI-1640 (Gibco, catalog number: 11875085)
3. DPBS (Gibco, catalog number: 14190136)
4. EDTA (Fisher Scientific, catalog number: AM9260G)
5. HEPES (Gibco, catalog number: 15630080)
6. L-Glutamine (Gibco, catalog number: 25030081)
7. Penicillin/streptomycin (Gibco, catalog number: 15070063)
8. Fetal bovine serum (FBS) (Gibco, catalog number: 16140071)
9. Teceleukin (recombinant human interleukin-2, IL-2) (Roche, catalog number: 23-6019)
10. PEG-it (System Biosciences, catalog number: LV810A-1)
11. DEAE dextran (Sigma-Aldrich, catalog number: D9885)
12. Cell culture lysis 5× reagent (Promega, catalog number: E153A)
13. Bright-Glo luciferase assay system (Promega, catalog number: E2610)
14. Glo lysis buffer (Promega, catalog number: E2661)
15. EasySepTM Human Memory CD4+ T-Cell Enrichment kit (STEMCELL Technologies, catalog number: 19157)
16. EasySepTM Human Memory CD8+ T-Cell Enrichment kit (STEMCELL Technologies, catalog number: 19159)
17. EasySepTM Human CD8 Positive Selection kit II (STEMCELL Technologies, catalog number: 17853) (optional)
18. UltraComp eBeadsTM compensation beads (Life Technologies, catalog number: 01-2222-42)
19. CountBright absolute counting beads (Invitrogen, catalog number: C36950)
20. Viability Marker Live/Dead Fixable aqua Dead Cell Stain kit (Invitrogen, catalog number: L34957) and anti-human antibodies for flow cytometry: PerCP/Cyanine5.5 CD45 (BioLegend, catalog number: 304028), BV785 CD3 (BioLegend, catalog number: 344842), BV421 CD4 (BioLegend, catalog number: 300532), BV711 CD8 (BioLegend, catalog number: 301043), AF700 CD45RO (BioLegend, catalog number: 304218), BV605 CD56 (BioLegend, catalog number: 318334), APC CD14 (BioLegend, catalog number: 367118), AF488 CD16 (BioLegend, catalog number: 302019), and PE CD19 (BioLegend, catalog number: 302208). The selection of viability maker and conjugated antibodies is dependent on the flow cytometer used for analysis and its laser/filter configurations
21. RBC lysis buffer/fixation solution (10×) (BioLegend, catalog number: 422401)
22. Hanks balanced salt solution (HBSS) (Gibco, catalog number: 14025076)
23. DMSO (Thermo Scientific Chemicals, catalog number: J66650.AK)
24. QIAamp Viral RNA Mini kit (250) (QIAGEN, catalog number: 52906)
25. AgPath-IDTM One-Step RT-PCR reagents (Thermo Scientific, catalog number: 4387391)
26. Forward primer (Integrated DNA Technologies, 5’-CGGGTTTATTACAGGGACA-3')
27. Reserve primer (Integrated DNA Technologies, 5’-C ACAATCATCACCTGCCAT-3')
28. ISCA probe (Integraated DNA Technologies, 5’-6FAM-AAAGGTGAAGGGGCAGTAGTAATACA-BHQ1-3’)
29. Tenofovir (TDF) (Gilead Sciences)
30. Emtricitabine (FTC) (API Sciences)
31. Dolutegravir (DTG) (Gilead Sciences)
32. Kleptose HPB (w/w) (Roquette, CAS number: 128446-35-5)
33. 4% Paraformaldehyde (PFA) solution (ChemCruz, catalog number: sc-281692)
34. Opti-MEM (Gibco, catalog number: 31985062)
35. FuGENE 6 transfection reagent (Promega, catalog number: E2691)
Solutions
1. D10 (see Recipes)
2. R10 (see Recipes)
3. R10-50 (see Recipes)
4. MACS buffer (see Recipes)
5. Freezing media (see Recipes)
6. Tenofovir (TDF), Emtricitabine (FTC), and Dolutegravir (DTG) antiretrovirals (see Recipes)
Recipes
1. D10
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM | n/a | 1,000 mL |
| FBS | 10% | 100 mL |
| HEPES | 1% | 10 mL |
| Penicillin/streptomycin | 1% | 10 mL |
2. R10
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| RPMI-1640 | n/a | 1,000 mL |
| FBS | 10% | 100 mL |
| HEPES | 1% | 10 mL |
| L-Glutamine | 1% | 10 mL |
| Penicillin/streptomycin | 1% | 10 mL |
3. R10-50
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| RPMI-1640 | n/a | 1,000 mL |
| FBS | 10% | 100 mL |
| HEPES | 1% | 10 mL |
| L-Glutamine | 1% | 10 mL |
| Penicillin/streptomycin | 1% | 10 mL |
| IL-2 | 50 units/mL | n/a |
4. MACS buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DPBS | n/a | 1,000 mL |
| FBS | 20% | 20 mL |
| EDTA | 2 mM | n/a |
5. Freezing media
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| FBS | 90% | 0.9 mL |
| DMSO | 10% | 0.1 mL |
6. Tenofovir (TDF), Emtricitabine (FTC), and Dolutegravir (DTG) antiretroviral (ARV) cocktail
Prepare 15% Kleptose HPB (w/w) in water. Add 80 mL of 15% Kleptose to a volumetric flask and stir in 250 mg of DTG-free base. Stir and sonicate for 30 min or until soluble. Add 510 mg of TDF and 4,000 mg of FTC to the same flask and stir for 3 min. Fill to 100 mL with 15% Kleptose. Confirm the pH is 4.2, filter sterilize through a 0.22 μM filter, and store aliquots at -20 °C until ready for use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 15% Kleptose HPB (w/w) | n/a | 100 mL |
| TDF | 5.1 mg/mL | 510 mg |
| FTC | 40.0 mg/mL | 4,000 mg |
| DTG | 2.5 mg/mL | 250 mg |
Laboratory supplies
1. Nunc T75 tissue culture flask (Thermo Scientific, catalog number: 156499)
2. 0.45 μm filter (Corning, catalog number: 431220)
3. 0.22 μm filter (Millipore Sigma, catalog number: SE1M179M6)
4. 96-well black/clear bottom plate, TC surface (Thermo Scientific, catalog number: 165305)
5. Nunc 15 mL conical sterile centrifuge tubes (Thermo Scientific, catalog number: 339650)
6. Nunc 50 mL conical sterile centrifuge tubes (Thermo Scientific, catalog number: 339652)
7. Alcohol prep pad (Mckensoon, catalog number: 191089)
8. Gauze pads 2 × 2 (Fisher Scientific, catalog number: 50-118-0367)
9. Surgical blade stainless steel No. 10 sterile (McKesson, catalog number: 1029067)
10. Microvette® 100 blood collection tubes (potassium EDTA 100/pk) (Kent Scientific, catalog number: MCVT100-EDTA)
11. Kwik-Stop® styptic powder with benzocaine (ARC International, catalog number: B000093HIR)
12. Cotton-tipped applicator swab stick, sterile, wood shaft (McKesson, catalog number: 24-106-2S)
13. Lo-DoseTM U-100 syringes (28G needle) (BD Biosciences, catalog number: 329461)
14. MicroAmp fast optical 96-well reaction plate, 0.1 mL (Applied Biosystems, catalog number: 4346907)
15. MicroAmp optical adhesive strip (Applied Biosystems, catalog number: 4311971)
16. 1.1 mL microtubes (Thermo Scientific, catalog number: 15086)
Equipment
1. CO2 incubator (Fisher Scientific, catalog number: 13-998-253)
2. Cell culture laminar flow hood, vacuum suction, temperature-controlled centrifuge, cell counter
3. SpectraMax i3x (Molecular Devices, catalog number: 10014-924) or equivalent
4. Mouse housing and handling facility
5. Heat lamp with base (Braintree Scientific, catalog number: HL-1B)
6. Rotating tail injector/restrainer (Braintree Scientific, catalog number: RTI)
7. EasySepTM magnet (for cell isolation) (STEMCELL Technologies, catalog number: 18001)
8. Attune NxT flow cytometer (Invitrogen, catalog number: A24858) or other flow cytometer
9. QIAcube HT (Qiagen, catalog number: 9001896) (optional)
10. QuantStudio 7 Pro Real-Time PCR System (Applied Biosystems, catalog number: A43183) or equivalent
11. T-Flex® Plus small animal handling glove (Lomir Biomedical Inc., catalog number: HG013G7)
Software and datasets
1. FlowJo (version 10.10), requires a license
2. Design and Analysis 2 (DA2) software (version 2.8.0), free
Procedure
文章信息
稿件历史记录
提交日期: Dec 13, 2024
接收日期: Feb 25, 2025
在线发布日期: Mar 10, 2025
出版日期: Apr 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Falling Iversen, E., Miller, I. G., Søgaard, O., Danesh, A. and Jones, B. R. (2025). A Participant-Derived Xenograft Mouse Model to Decode Autologous Mechanisms of HIV Control and Evaluate Immunotherapies. Bio-protocol 15(7): e5254. DOI: 10.21769/BioProtoc.5254.
分类
免疫学 > 动物模型 > 小鼠
细胞生物学 > 细胞移植 > 异种移植
免疫学 > 免疫疗法
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link