- EN - English
- CN - 中文
Evaluating In Vivo Translation Inhibition via Puromycin Labeling in Botrytis cinerea Germlings Treated With Antifungal Peptides
嘌呤霉素标记评估抗真菌肽对灰葡萄孢萌发菌丝的体内翻译抑制作用
发布: 2025年03月20日第15卷第6期 DOI: 10.21769/BioProtoc.5250 浏览次数: 2522
评审: Ritu GuptaYuko KuritaAnonymous reviewer(s)

相关实验方案

基于 Emu 的可重复计算流程:在高性能计算集群上实现高保真土壤—植物微生物组解析
Henrique M. Dias [...] Christopher Graham
2026年01月20日 439 阅读
Abstract
Antimicrobial peptides are effective agents against various pathogens, often targeting essential processes like protein translation to exert their antimicrobial effects. Traditional methods such as puromycin labeling have been extensively used to measure protein synthesis in mammalian and yeast systems; however, protocols tailored for plant pathogenic filamentous fungi, particularly those investigating translation inhibition by antifungal peptides, are lacking. This protocol adapts puromycin labeling to quantify translation inhibition in Botrytis cinerea germlings treated with antifungal peptides. Optimizing the method specifically for fungal germlings provides a precise tool to investigate peptide effects on fungal protein synthesis, advancing our understanding of translation dynamics during pathogen–host interactions in filamentous fungi.
Key features
• This protocol is designed for in vivo experiments, enabling the estimation of protein translation inhibition by antifungal peptides in Botrytis cinerea.
• It is optimized for antifungal peptides capable of penetrating fungal cells and inhibiting translation, making it applicable to other filamentous fungal pathogens.
• The protocol is ideal for laboratories utilizing traditional western blotting techniques, ensuring accessibility and ease of implementation.
• This protocol for in vivo application in filamentous fungi is adapted from the SUnSET method [1].
Keywords: Puromycin labeling (嘌呤霉素标记)Graphical overview
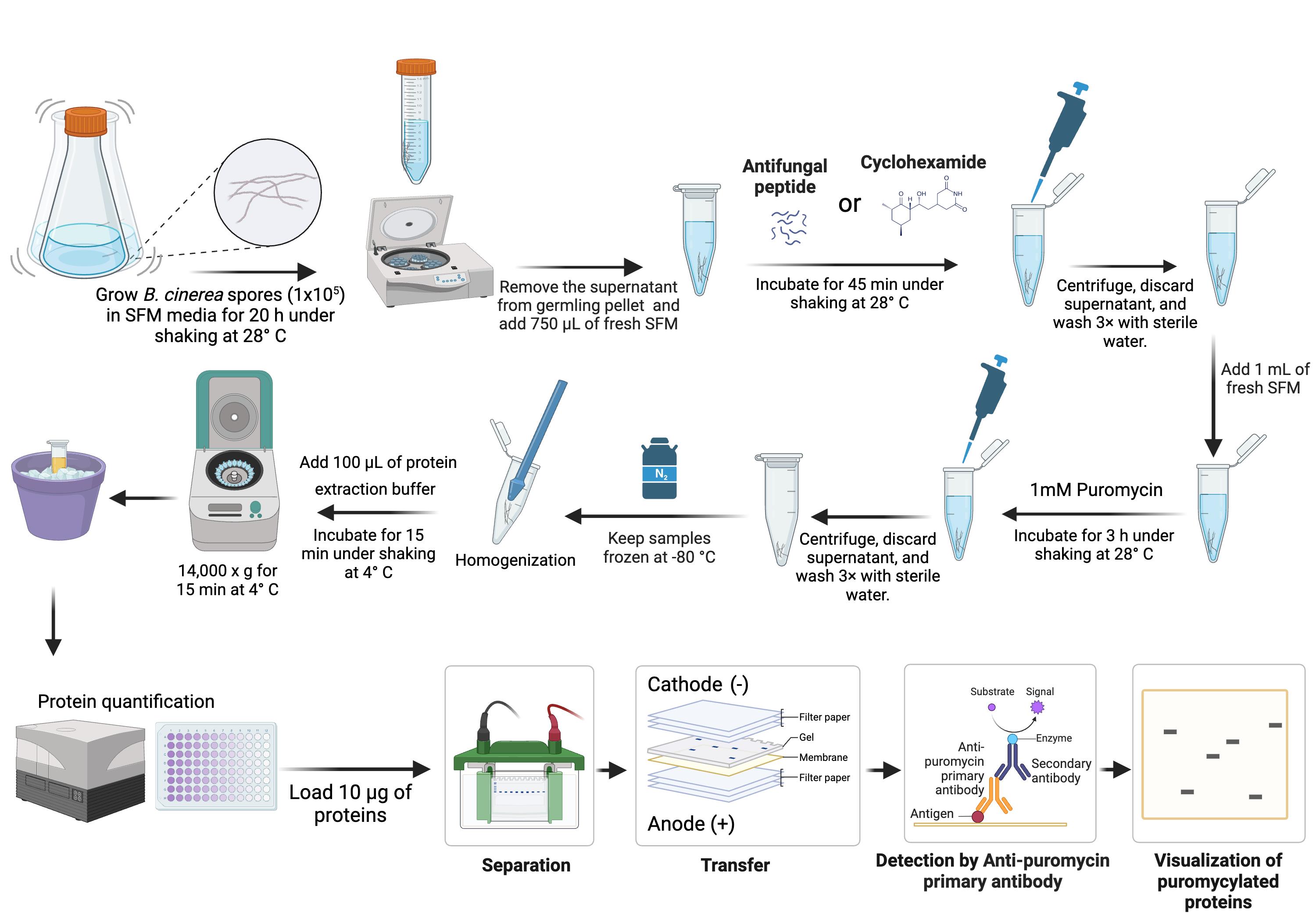
Background
Antifungal peptides have emerged as powerful agents in the fight against fungal pathogens [2]. These peptides are particularly promising in agriculture, where fungal pathogens cause significant crop losses [3]. Understanding their modes of action, such as their inhibitory effects on fungal protein synthesis, is critical for developing innovative and sustainable strategies to manage fungal diseases. Among existing methodologies, puromycin labeling has been extensively used in mammalian and yeast systems to measure protein synthesis. Puromycin, an aminoacyl-tRNA analog, is selectively incorporated into nascent polypeptides on actively elongating ribosomes [4]; puromycin labeling exploits puromycin’s ability to be incorporated into elongating polypeptide chains, providing a direct and reliable assessment of translation activity. However, protocols specifically designed to study protein translation inhibition in filamentous fungal pathogens, such as Botrytis cinerea, are lacking. Filamentous fungi present unique challenges due to their complex cellular structures, growth patterns, and physiological processes, which differ significantly from those of yeast and mammalian cells. As a result, traditional puromycin labeling protocols often require substantial adaptation to suit the biology of these organisms. By adapting puromycin labeling to B. cinerea, this protocol enables the precise quantification of protein translation inhibition following treatment with previously characterized antifungal peptide disulfide variants, such as NCR13_PFV1 and NCR13_PFV2 [5]. Prior work from our lab [5] has demonstrated that NCR13_PFV1 exhibits stronger translation inhibition than NCR13_PFV2 in a wheat germ in vitro translation system [6]. However, the in vivo translation-inhibitory effects of these peptides in B. cinerea remain to be determined. To address this, we have developed and optimized an in vivo protocol specifically tailored for B. cinerea germlings, enabling precise measurement of translation inhibition by antifungal peptides.
This protocol offers a few key advantages over existing methodologies. First, it is designed for the model fungus B. cinerea germlings, ensuring high sensitivity and reproducibility in measuring translation dynamics. Second, it provides a direct measure of protein translation inhibitory effects of antifungal peptides, allowing researchers to extend its use to other filamentous fungal pathogens. This protocol is also suitable for the identification of novel antifungal compounds that target protein translation in vivo. However, it also has limitations, including the need for further optimization for different fungal species or developmental stages and the potential variability in germling responses across isolates. By addressing a critical methodological gap, this protocol will allow us to explore novel antifungal mechanisms and contribute to the development of effective strategies for managing fungal diseases in agriculture and beyond.
Materials and reagents
Biological materials
1. Pathogen: B. cinerea strain T4 [5]. B. cinerea spores are preserved in 25% glycerol at -80 °C for long-term storage. B. cinerea can be obtained from the American Type Culture Collection (ATCC)
2. Peptides: Nodule-Specific Cysteine-Rich 13 (NCR13) peptides: NCR13_PFV1 and NCR13_PFV2. Details of peptide synthesis and characterization of NCR13_PFV1 and NCR13_PFV2 can be found in [5]. These peptides are stored at -20 °C, and the required peptide concentrations are prepared in sterile Milli-Q water
Reagents
1. Puromycin (Cell Signaling Technology, catalog number: 40939S)
2. 1 M HEPES (Thermo Scientific, catalog number: J61275)
3. EDTA (Sigma-Aldrich, catalog number: E5134)
4. ProBlock gold yeast/fungi protease inhibitor cocktail (GoldBio, catalog number: GB-333-1)
5. 4%–20% mini-PROTEAN TGX stain-free protein gel (Bio-Rad, catalog number: 4568094
6. Bovine serum albumin (BSA) (Goldbio, catalog number: A-421-100)
7. ProSieve QuadColor protein ladder (VWR, catalog number: 89125-526)
8. Cycloheximide (CHX) (Sigma-Aldrich, catalog number: 239763)
9. Mouse anti-puromycin antibody (clone 12D10) (Merck Millipore, catalog number: MABE343)
10. HRP-conjugated anti-mouse secondary antibody (Thermo Fisher Scientific, catalog number: G-21040)
11. Pierce BCA Protein Assay kit (Thermo Scientific, catalog number: 23227)
12. SuperSignal West PICO PLUS chemiluminescent substrate (Thermo Scientific, catalog number: 34580)
13. Triton X-100 (Sigma-Aldrich, catalog number: T8787)
14. V8 vegetable juice (Campbell Soup)
15. Calcium carbonate (Sigma-Aldrich, catalog number: C6763)
16. Potassium hydrogen phosphate (K2HPO) (G-Biosciences, catalog number: RC-081)
17. Magnesium sulfate (MgSO4) (GoldBio, catalog number: 10034-99-8)
18. Ferrous sulfate (FeSO4) (Sigma-Aldrich, catalog number: 12354)
19. Cobalt(II) chloride (CoCl2) (Acros Organics, catalog number: 423571000)
20. Copper(II) sulfate (CuSO4) (Sigma-Aldrich, catalog number: C3036)
21. Sodium molybdate (NaMoO4) (Sigma-Aldrich, catalog number: 243655)
22. Boric acid (HBO) (Sigma-Aldrich, catalog number: B6768)
23. Potassium iodide (KI) (Sigma-Aldrich, catalog number: 746428)
24. Zinc sulfate (ZnSO4) (Sigma-Aldrich, catalog number: Z0501)
25. Manganese (II) sulfate (MnSO4) (Thermo Scientific, catalog number: 033341-36)
26. L-Methionine (Sigma-Aldrich, catalog number: M5308)
27. myo-Inositol (Sigma-Aldrich, catalog number: I7508)
28. Biotin (Sigma-Aldrich, catalog number: B4501)
29. Thiamine hydrochloride (Thermo Scientific, catalog number: 148991000)
30. Pyridoxine hydrochloride (Sigma-Aldrich, catalog number: P8666)
31. D-Glucose (Research Products International, catalog number: G32040)
32. L-Asparagine (Bioworld, catalog number: 40100280)
33. Glycerol (Thermo Scientific, catalog number: 158922500)
34. Bacto agar (Fisher Scientific, catalog number: DF0140-01-0)
35. 2-Mercaptoethanol (Sigma-Aldrich, catalog number: M6250)
36. Tris base (Sigma-Aldrich, catalog number: 648310)
37. Glycine (Amresco, catalog number: M103)
38. Methanol (VWR, catalog number: 0323-4L)
39. Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 567530)
40. Phosphate buffered saline (PBS), 10× (Thermo Scientific, catalog number: BP39920)
41. Tween 20 (Sigma-Aldrich, catalog number: P1379)
42. Laemmli protein sample buffer 4× (Bio-Rad, catalog number: 1610747)
Solutions
1. 2× Synthetic fungal media (SFM) (see recipes)
2. Protein extraction buffer (see recipes)
3. PBST buffer (see recipes)
4. Western blot (WB) transfer buffer (see recipes)
5. Puromycin stock preparation (see recipes)
6. Cycloheximide stock preparation (see recipes)
7. V8 media (see recipes)
8. 0.5 M EDTA solution (see recipes)
Recipes
1. 2× ynthetic fungal media (SFM) (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Glucose | 20 g/L | 10 g |
| Asparagine | 2 g/L | 1 g |
Dissolve glucose and asparagine in the appropriate volume (474.62 mL) of distilled water and autoclave this solution at 121 °C for 20 min.
After cooling, add the following stock solutions of vitamins, trace elements, and other supplements to the autoclaved solution.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M K2HPO4 | 5 mM | 2.5 mL |
| 0.5 M MgSO4 | 100 μM | 100 μL |
| 0.05 M FeSO4 | 10 μM | 10 μL |
| 0.01 M CoCl2 | 0.2 μM | 10 μL |
| 0.01 M CuSO4 | 0.2 μM | 10 μL |
| 0.05 M Na2MoO4 | 4 μM | 10 μL |
| 0.05 M H3BO3 | 1 μM | 10 μL |
| 0.01 M KI | 0.2 μM | 10 μL |
| 0.05 M ZnSO4 | 1 μM | 10 μL |
| 0.01 M MnSO4 | 0.2 μM | 10 μL |
| 2 g/L Methionine | 40 mg/L | 10 mL |
| 2 g/L Myo-inositol | 4 mg/L | 1 mL |
| 2 g/L Biotin | 0.4 mg/L | 100 μL |
| 2 g/L Thiamine HCl | 2 mg/L | 500 μL |
| 2 g/L Pyridoxine HCl | 0.4 mg/L | 100 μL |
After adding all components, filter sterilize the solution using a bottle-top vacuum filter (0.22 μM). Store the SFM medium at 4 °C until use. The SFM medium has a shelf life of up to 3 months. Dilute in sterile Milli-Q water as necessary.
2. Protein extraction buffer (1 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M HEPES buffer | 100 mM | 100 μL |
| 0.5 M EDTA | 5 mM | 10 μL |
| 100% Triton-X 100 | 0.1% | 1 μL |
| Protease inhibitor cocktail | 1× | 10 μL |
| Milli-Q water | Make up the volume to 1 mL | 880 μL |
Prepare fresh protein extraction buffer for each extraction. Store at 4 °C until use.
3. PBST buffer (200 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× PBS stock solution | 1× | 20 mL |
| Tween-20 | 0.1% (v/v) | 0.2 mL |
| Milli-Q water | Make up the volume to 200 mL | 179.8 mL |
4. Western blot (WB) transfer buffer (200 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 25 mM | 0.605 g |
| Glycine | 192 mM | 2.88 g |
| Methanol | 20% (v/v) | 40 mL |
| Milli-Q water | Make up the volume to 200 mL | 159.4 mL |
5. Puromycin stock preparation
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Puromycin | 50 mM | 27.5 mg in 1 mL |
Freshly prepare in sterile Milli-Q water and keep at -20 °C in aliquots.
6. Cycloheximide stock preparation
| Reagent | Stock concentration | Quantity or Volume |
|---|---|---|
| Cycloheximide | 177.6 μM | 50 µg in 1 mL |
Freshly prepare in sterile Milli-Q water and keep at -20 °C in aliquots.
7. V8 media (1000 mL)
| Reagent | Quantity or Volume |
|---|---|
| V8 juice | 200 mL |
| Calcium carbonate | 3 g |
| Bacto agar | 15 g |
| Total | Make up the volume to 1000 mL |
8. 0.5 M EDTA solution (500 mL)
| Reagent | Quantity or Volume |
|---|---|
| EDTA | 93.05 g |
Dissolve in 350 mL of Milli-Q water. Adjust the pH to 8.0 with NaOH (~10 g of NaOH pellets). Make up the volume to 500 mL using Milli-Q water. Dispense into aliquots and sterilize by autoclaving.
Equipment
1. Mini-PROTEAN tetra vertical electrophoresis cell (Bio-Rad, catalog number: 1658004)
2. Trans-Blot Turbo transfer system (Bio-Rad, catalog number: 1704150)
3. ChemiDoc touch imaging system (Bio-Rad, catalog number: 12003153)
4. Pipettes and micropipettes (Gilson)
5. Centrifuge (Eppendorf, model: 5810R)
6. Cold room
7. Heat block (Fisher Scientific, catalog number: 117186)
8. Pestles (Kimble Chase, catalog number: G3956477)
9. Bottle-top vacuum filter (Corning, catalog number: 430521)
10. Improved Neubauer hemocytometer (Grid Optik, model: JSB348)
11. Nitrocellulose membrane (Cytiva, catalog number: 10600009)
12. Pipette aspirator (Biologix, catalog number: 01-2201)
13. Incubator Shaker (New Brunswick Scientific, model: I26)
14. Rotator (Labnet International, catalog number: H5500)
15. Orbital shaker (VWR, model: MP4)
16. Light microscope (Olympus, model: CKX31)
17. SterilGard III Advance Biosafety cabinet (Baker, model number: SG603A)
18. Autoclave (Beta Star)
Software and datasets
1. BioRender (https://www.biorender.com/). The following figures were created using BioRender: https://app.biorender.com/illustrations/66a3f08c8d303af25d573a57
Procedure
文章信息
稿件历史记录
提交日期: Dec 23, 2024
接收日期: Feb 17, 2025
在线发布日期: Mar 9, 2025
出版日期: Mar 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Godwin, J. and Shah, D. M. (2025). Evaluating In Vivo Translation Inhibition via Puromycin Labeling in Botrytis cinerea Germlings Treated With Antifungal Peptides. Bio-protocol 15(6): e5250. DOI: 10.21769/BioProtoc.5250.
分类
微生物学 > 抗微生物试验 > 抗真菌试验
植物科学 > 植物免疫 > 宿主-细菌相互作用
分子生物学 > 蛋白质 > 抗微生物分析
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











