- EN - English
- CN - 中文
Generation of FECD Phenotypes in the Mouse Cornea by UVA Exposure and Surgical Removal of its Corneal Endothelial Layer
通过 UVA 照射和角膜内皮层手术去除在小鼠角膜中构建 FECD 表型
发布: 2025年03月20日第15卷第6期 DOI: 10.21769/BioProtoc.5249 浏览次数: 2678
评审: Pilar Villacampa AlcubierreDogan Can KirmanGülçin ÇAKAN AKDOĞAN

相关实验方案

蚊虫和白蛉中肠组织的优化解离方法:用于高质量单细胞RNA测序
Ana Beatriz F. Barletta [...] Carolina Barillas-Mury
2025年06月20日 2415 阅读

髓系肿瘤中用于生殖系 DNA 分离的皮肤成纤维细胞标准化培养:一种从床旁到实验室的实用方法
Parampreet Kour [...] Pulkit Rastogi
2025年10月05日 1418 阅读
Abstract
Fuchs endothelial corneal dystrophy (FECD) is a rare and multifactorial disorder leading to cell death in the innermost layer of the cornea, i.e., the endothelium; UV radiation is reported as the major environmental risk for the disease. Establishing an animal model for this disease has remained challenging in FECD research. We have developed a detailed protocol for the establishment of a UVA-induced FECD mouse model and removal of corneal endothelium from the eye for further molecular and histological studies by taking references from previous studies. UVA light of 500 J/cm2 was focused on the C57BL/6J female mouse cornea and kept for an observation period of 90 days. The animal developed corneal scarring by the end of three months. Slit-lamp microscopy and alizarin red–trypan blue staining confirmed endothelial cell death and formation of corneal guttae in the endothelium. Surgical removal of the endothelial layer was successfully done in the diseased mouse, and the result was confirmed by immunofluorescence. This study is relevant for in-depth research using a FECD mouse model, which will surpass the limitation of human tissue scarcity and can be used for in vivo drug targeting to develop therapeutics to cure FECD.
Key features
• UVA radiation induces FECD only in the exposed eye of female mice.
• Females are more affected and develop the FECD phenotype.
• This protocol will help dissect the endothelium layer with Descemet’s membrane (DM) from the mouse cornea, which is equivalent to human surgical tissue.
Keywords: Cornea (角膜)Graphical overview
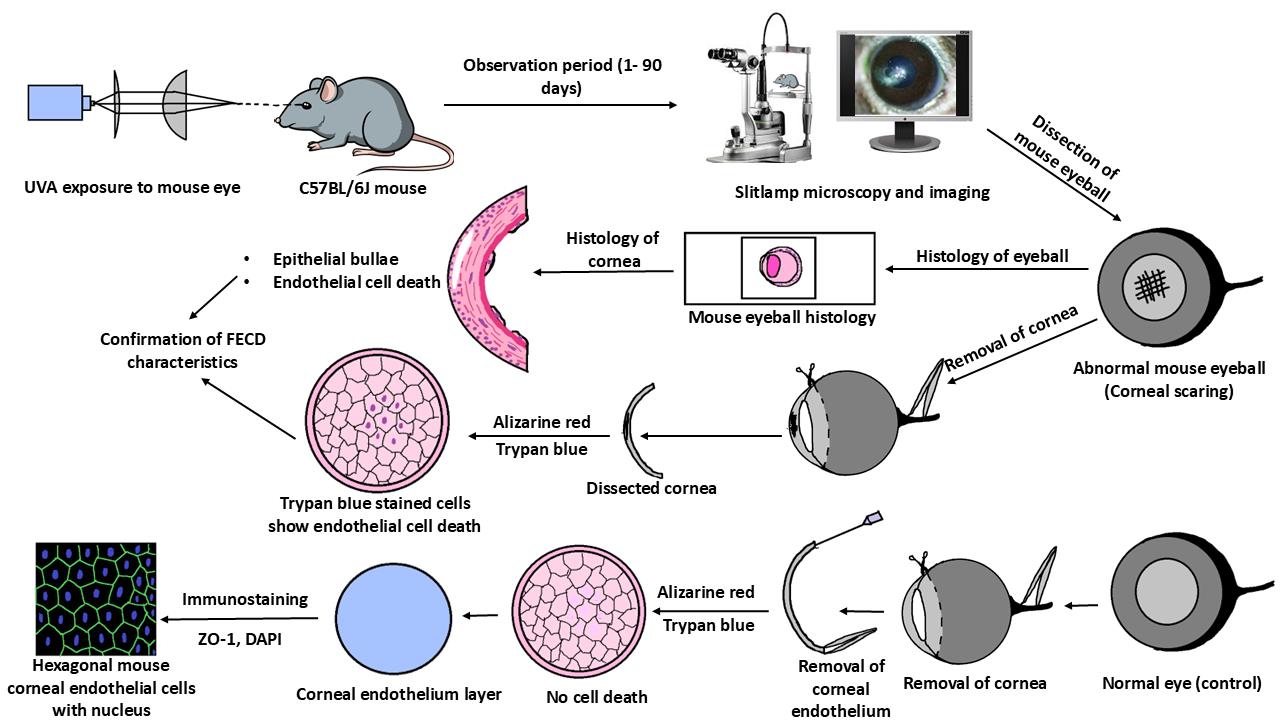
Background
Fuchs endothelial corneal dystrophy (FECD) is an age-related, rare corneal disease resulting in the loss of corneal endothelial cells from the cornea's innermost layer. As the cornea is avascular, these endothelial cells aid in the transport of nutrients and water between the corneal stroma and the aqueous humor of the eye [1]. Loss of these cells leads to various FECD phenotypes such as guttae formation, thickening of Descemet’s membrane (DM) due to extracellular matrix protein (ECM) deposition, disruption of stromal collagen fibers due to water retention, and, in severe cases, epithelial bullae formation [2,3].
As per the age of onset, the disease is of two types, i.e., early-onset form (<40 years of age) and late-onset form (>40 years of age). As per its rare status, the late-onset form is more common than the early-onset form [4]. Moreover, the disease pervasiveness varies in different populations. The highest percentage of diseased individuals was reported in the Caucasian population (3.9% of Americans, 11.0% of women and 7.0% of men in Iceland, 3.7% of Japanese, and 6.6% of the Chinese Singaporean population). The LV Prasad Eye Institute (LVPEI), in Hyderabad, operated 7.5%–10.4% of FECD cases within a period of 5 years, i.e., from 2007–2011 [5–9]. The rare status of the FECD disease acts as a limitation for in-depth research in this field to better understand the disease pathology and develop a drug to treat the disease. This limitation can be overcome by generating an animal model for FECD. Albert S. Jun and his group generated a mouse model for early-onset FECD with a point mutation substituting glutamine for lysine (Q455K) [10]. Subhashree Murugan and collaborators developed a mouse model with a Q455K mutation along with SLC4A11 knockdown, which showed the phenotypes of FECD [11]. Liu et al., for the first time in 2020, developed a mouse model for late-onset FECD by exposing the mouse eye to ultraviolet A (UVA) radiation and validated the model with further experiments, but the detailed procedure is unavailable [12]. Hongmin Yun and collaborators developed a dissection method to separate the retina from the mouse eye to study innervation [13].
No specific studies describe the dissection method of mouse cornea from the eyeball and the further removal of the corneal endothelium layer from it. Our study details the process of generating a nongenetic FECD mouse model with UVA exposure, highly inspired by the study conducted by Liu et al., as UVA acts as an environmental risk factor for FECD causation. Further, we have adapted the initial technique from Yun et al. to separate the cornea and retina from the mouse eyeball and developed a new technique to remove the endothelium layer from the cornea. The model presented here will enable researchers to explore disease mechanisms and effects of various perturbations during FECD disease progression, using an in vivo model that replicates the disease phenotypes well. As the disease initially targets corneal endothelium, its removal from the cornea can be used in various molecular and biochemical experiments to answer many unknown and unrevealed questions in FECD pathogenesis and progression.
Materials and reagents
Biological material
1. C57BL/6J mouse (Nucleus breeding stock, The Jackson Laboratory, IMSR_JAX:000664)
Reagents
1. Carboxymethyl cellulose eye drop (Refresh Tears, catalog number: 119573)
2. Fluorescein powder (Himedia, catalog number: GRM374-100G)
3. PBS (Himedia, catalog number: TL1101)
4. Dimethyl sulfoxide (DMSO) (Himedia, catalog number: GRM5856)
5. Isoflurane I. P. (Sorsane®, catalog number: LUNP700K)
6. Trypan blue solution (Himedia, catalog number: TCL046)
7. Alizarin red (Himedia, catalog number: GRM894)
8. Paraformaldehyde (PFA) powder (Himedia, catalog number: TC703)
9. 100% ethanol (Himedia, catalog number: MB228)
10. Glacial acetic acid (SRL, catalog number: 85801)
11. Paraffin wax (Himedia, catalog number: GRM1042)
12. Xylene (SRL, catalog number: 90998)
13. Hematoxylin solution (Himedia, catalog number: S034)
14. Eosin solution (Himedia, catalog number: S0007)
15. ZO-1 antibody (Santa Cruz, catalog number: sc-10804)
16. Fluoromount G (Southern Biotech, catalog number: 0100–01)
17. Mili-Q water
18. RNAlater (Invitrogen, catalog number: AM7020)
19. 4', 6-Diamidine-2'-phenylindole dihydrochloride (DAPI) (Sigma, catalog number: 350 10236276001)
20. Normal horse serum (Thermo Scientific, catalog number: 31874)
21. Formaldehyde solution (Sigma-Aldrich, catalog number: 252549)
22. Alexa Fluor conjugated secondary antibody (Invitrogen, catalog number: A48254)
23. Triton X-100 (Sigma-Aldrich, catalog number: T8787)
24. Amrut feed (Krishna Valley Agrotech, catalog number: Breeding AF-6000B)
Solutions
1. 0.5% Fluorescein dye (see Recipes)
2. 4% PFA solution (see Recipes)
3. Hartman’s fixative solution (see Recipes)
4. 1% Alizarin Red solution (see Recipes)
Recipes
1. 0.5% Fluorescein dye (10 mL)
Note: This solution can be stored at room temperature for up to 15 days.
| Reagents | Final concentration | Final quantity or volume |
|---|---|---|
| Fluorescein | 5 mg/mL | 0.05 g |
| DMSO | NA | 10 mL |
2. 4% PFA solution (100 mL)
Note: This solution is prepared by dissolving PFA powder at 95 °C in a thermomixer. It can be stored for up to 7 days at 4 °C and up to 1 month at -20 °C.
| Reagents | Final concentration | Final quantity or volume |
|---|---|---|
| PFA | 40 mg/mL | 4 g |
| 1× PBS | NA | 100 mL |
3. Hartman’s fixative solution (20 mL)
Note: This solution can be stored for up to 1 month at 4 °C.
| Reagents | Final concentration | Final quantity or volume |
|---|---|---|
| Formaldehyde, 37% | 3 M | 4.44 mL |
| Glacial acetic acid, 99% | 2.22 mL | |
| Ethanol, 95% | 6.66 mL | |
| Mili-Q water | NA | 6.66 mL |
4. 1% Alizarin red solution (100 mL)
Note: Filter the solution and use it immediately with a 0.45 μm syringe filter.
| Reagents | Final concentration | Final quantity or volume |
|---|---|---|
| Alizarin red | 10 mg/mL | 1 g |
| 1× PBS | NA | 100 mL |
Laboratory supplies
1. Petri dish (Tarsons, catalog number: 460090)
2. Pasteur pipette (Tarsons, catalog number: 940050)
3. Syringe filter (filtration rating: 0.45 μm; diameter: 33 mm) (Axiva, catalog number: SFNY33XB)
4. 5 mL syringe with needle (Dispovan, catalog number: 13483)
5. 10 mL syringe with needle (Dispovan, catalog number: 13484)
6. Glass slide (Himedia, catalog number: BG003)
7. Coverslip (Himedia, catalog number: BG011C)
8. 50 mL Falcon tube (Tarsons, catalog number: 339652)
9. 15 mL Falcon tube (Tarsons, catalog number: 546021)
10. 1.5 mL tubes (Tarsons, catalog number: 500010)
11. Glass bottle with screw cap (Borosil, catalog number: 1506)
12. Zero-size painting brush (Camlin, catalog number: Round-66 series)
13. Pointed forceps (Medsor Impex)
14. Curved-end micro-dissecting forceps, 13 × 1 × 2 cm, 200 g (Medsor Impex, catalog number: 8903554956729, Amazon Standard Identification Number: B07FZWR13X)
15. Micro-suture cutting scissors, 1 × 1 × 1 cm, 0.28 g (Addler CE, model: DSCF2465, Amazon Standard Identification Number: B06X988149)
Equipment
1. LED light source 365 nm (Thorlabs, catalog number: M365LP1)
2. Stand for the light source (PHYWE, catalog number: 02002–55)
3. Optical collimator (Thorlabs, catalog number: SM1U25)
4. Planoconvex lens 1(Thorlabs, catalog number: LA1951-A)
5. Planoconvex lens 2 (Thorlabs, catalog number: LA1229-A)
6. Lens mount (Thorlabs, catalog number: LMR1)
7. Lens mount base (Thorlabs, catalog number: BA1L/M)
8. Lens stand holder (Thorlabs, catalog number: UPH50/M-P5)
9. Power meter with laser sensor (Ophir, catalog number: JUNO ROHS, part number: 7Z01250)
10. LED driver (Thorlabs, catalog number: DC2200)
11. Optical breadboard (Gaussian Optics, catalog number: 90019010)
12. UV/VIS detector card, 250–540 nm (Thorlabs, catalog number: VRC1)
13. UV protection glasses (Thorlabs, catalog number: LG6)
14. Laboratory jack (Labpro, catalog number: 756578)
15. Slit-lamp microscope with a digital imaging system (Appasamy Associates, catalog number: ICCM-1dynamIQ AIA- 11- 5)
16. Anesthesia system for isoflurane (VetEquip, catalog number: 161010)
17. Light microscope (Nikon, catalog number: SMZ25)
18. Confocal microscope (Leica, catalog number: SP8)
19. Microtome (Radical Scientific, catalog number: RMT-35)
20. Hot air oven (Remi, catalog number: RHI-80)
21. Gyro twister (Labnet, catalog number: S1000, A-B-230)
22. Thermomixer (Eppendorf, catalog number: 5382)
23. Individually ventilated caging (IVC) system (Orchid Scientific, model: US- Y- UV)
Procedure
文章信息
稿件历史记录
提交日期: Nov 15, 2024
接收日期: Feb 12, 2025
在线发布日期: Mar 9, 2025
出版日期: Mar 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Sharma, S., Sahoo, R., Chawla, S., Das, S. and Alone, D. P. (2025). Generation of FECD Phenotypes in the Mouse Cornea by UVA Exposure and Surgical Removal of its Corneal Endothelial Layer. Bio-protocol 15(6): e5249. DOI: 10.21769/BioProtoc.5249.
分类
细胞生物学 > 组织分析 > 组织分离
医学 > 眼科
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link










