- EN - English
- CN - 中文
Protocol for Inoculation of PGPR Staphylococcus sciuri to Seeds and Seedlings of Rice and Tomato Plants for Increased Root and Shoot Growth
PGPR 金黄色葡萄球菌接种水稻和番茄种子及幼苗的方案以促进根系和茎叶生长
发布: 2025年03月20日第15卷第6期 DOI: 10.21769/BioProtoc.5241 浏览次数: 2201
评审: Anonymous reviewer(s)

相关实验方案

将Miniprep制备的大肠杆菌K12菌株质粒DNA转化为可用于植物遗传转化的根癌农杆菌EHA105细胞的简单可靠方法
Beenzu Siamalube [...] Steven Runo
2025年01月05日 2374 阅读
Abstract
Plant growth–promoting rhizobacteria (PGPR) can be used as biofertilizers to enhance crop growth for better yield and soil fertility restoration. PGPR possesses certain traits such as nutrient solubilization, phytohormone production, and production of key enzymes for improved crop growth. These traits are also important for inhibiting the growth of plant root pathogens, improving root development, and conferring stress tolerance. However, the mere presence of PGPR traits in isolated bacteria may not directly reflect an improvement in plant growth, warranting researchers to evaluate phenotypic and physiological changes upon inoculation. The current manuscript provides a detailed step-by-step procedure for inoculating the PGPR Staphylococcus sciuri into seeds and seedlings of rice and tomato plants for visualizing the enhancement of root and shoot growth. The surface-sterilized seeds of rice and tomato plants are inoculated overnight with an actively grown log-phase culture of S. sciuri, and differences in growth and biomass of seedlings that emerged from the inoculated and uninoculated seeds are analyzed 10 days after germination. Plants grown in pots with sterile soil are also treated with PGPR S. sciuri by soil drenching. A remarkable increase in root and shoot growth is observed in inoculated plants. We suggest that treating seeds with bacteria and enriching the soil with bacterial inoculum provides an adequate load of PGPR that facilitates growth improvement. This method can be a reliable choice for screening and evaluating plant growth promotion by either isolated bacteria or bacterial consortia with plant-beneficial traits.
Key features
• Inoculation of seeds and seedlings with an actively grown log-phase culture of S. sciuri can induce root and shoot growth in rice and tomato plants.
• The improvement of root and shoot growth due to inoculation with S. sciuri can be evaluated under laboratory conditions.
• The method can be used to screen bacteria with plant growth promotion ability under laboratory conditions.
• The method is robust and can be used for evaluating the plant growth–promoting ability of many bacteria and the inoculation of multiple plant species.
Keywords: Plant growth–promoting rhizobacteria (植物生长促进根际菌)Graphical overview
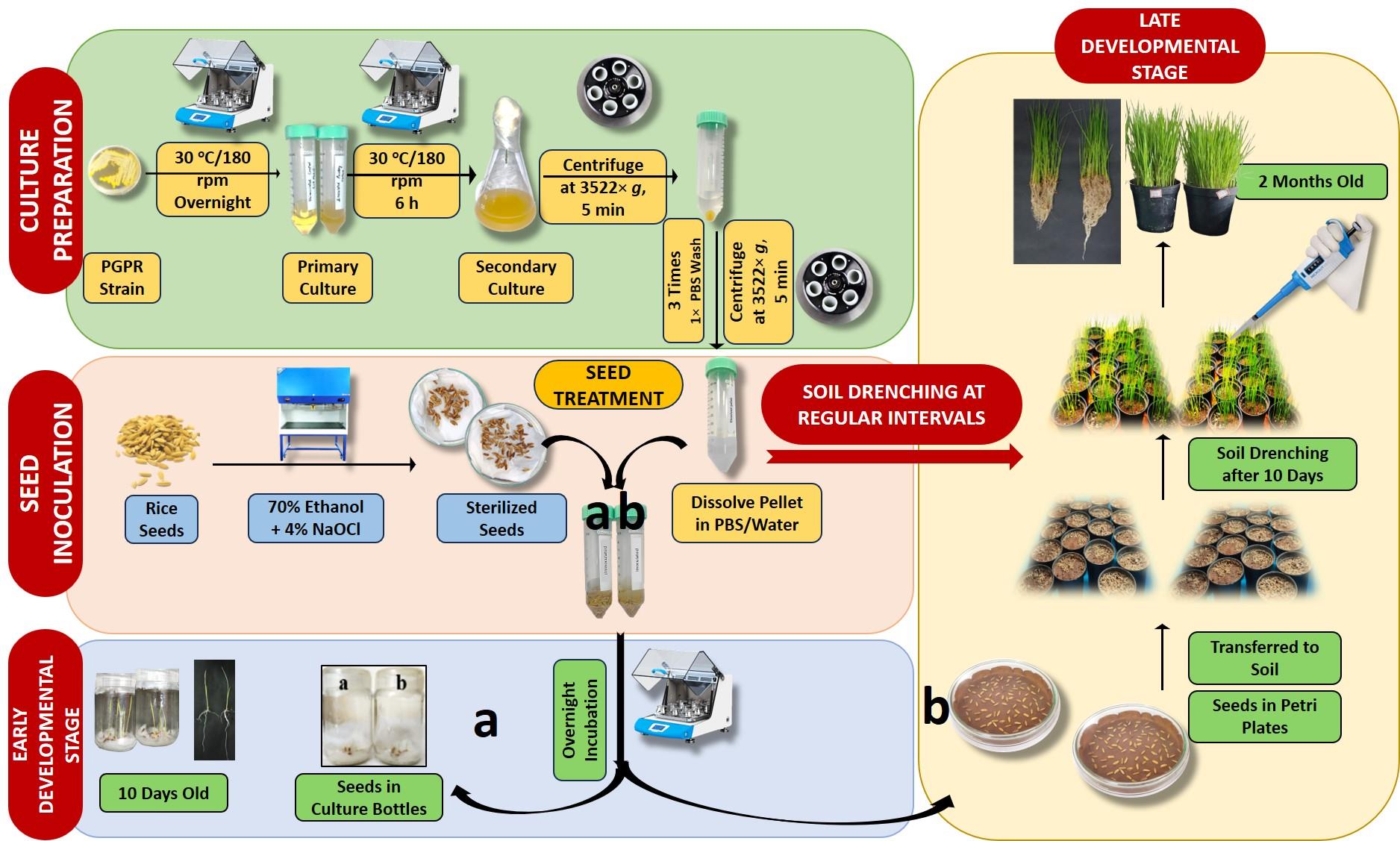
Graphical overview of inoculation of seeds and seedlings of rice plants with plant growth–promoting rhizobacteria (PGPR) for increased root and shoot growth
Background
Among the vast majority of microorganisms found in soil, plant growth–promoting rhizobacteria (PGPR) colonize the rhizosphere or grow inside roots and have the ability to improve plant growth under normal and stress conditions [1–3]. PGPR offer various benefits to host plants, including increased nutrient availability, modulation of phytohormone production, mitigation of biotic and abiotic stresses, protection from pathogens, and improved plant growth [3,4]. Bacteria with plant growth–promoting traits can be isolated from different soil types and ecosystems, but evaluating this ability in isolated bacteria requires the use of a reliable and stable method for inoculation. As per the literature, PGPR inoculation is performed on seeds by coating, treating the soil with bacteria, soil drenching, or application of bacteria via foliar spray [5,6]. All these treatments are proven to be effective in certain crops with specific advantages and disadvantages [7]. Often, the bacterial load and the survival of the bacteria in the soil are questioned [8]. Therefore, a reliable and robust inoculation protocol is required that can be used for bacterial inoculation on multiple crop species and for screening the plant growth–promoting ability of multiple bacteria or consortia [7,9]. A successful bacterial inoculation depends on various factors like the inoculation technique, density of inoculum given to the host crop, bacteria effectiveness in root colonization, soil humidity, pH, temperature, developmental stage of the plant being inoculated, and root exudate status [10,11]. In the current protocol, a combination of seed inoculation and soil drenching is described for assessing increased root and shoot growth of Oryza sativa and Lycopersicon esculentum plants.
Materials and reagents
Biological material
1. Bacterial strain Staphylococcus sciuri ET101, halotolerant PGPR isolated from the rhizosphere of Salicornia species
2. Oryza sativa (Aiswarya cultivar), purchased from Regional Agricultural Research Station, Pattambi, Kerala, India
3. Lycopersicon esculentum (PKM seeds), purchased from Sri Venkateswara Agro Store, Tiruchirappalli, Tamil Nadu, India
Reagents
1. LB agar (SRL, catalog number: LM018)
2. LB broth (Himedia, catalog number: M1245)
3. Absolute ethanol (Analytic CS Reagent, catalog number: 1170)
4. Sodium hypochlorite (Nice, catalog number: S2195)
5. KCl (Merck, catalog number: 1.93238.0521)
6. NaCl (Merck, catalog number: 1.93606.0521)
7. Na2HPO4 (Merck, catalog number: 1.93209.0521)
8. KH2PO4 (Merck, catalog number: 1.93205.0521)
9. NaOH (SRL, catalog number: 68151)
10. HCl (Finar, catalog number: 70770L8M50)
Solutions
1. 10× phosphate-buffered saline (PBS) (see Recipes)
Recipes
1. 10× phosphate-buffered saline (PBS)
| Reagents | Final concentration |
|---|---|
| NaCl | 1.37 M |
| KCl | 27 mM |
| Na2HPO4 | 100 mM |
| KH2PO4 | 18 M |
| Sterile distilled water | Make up to 1 L |
Adjust the pH to 7.4 with 1N NaOH or 1N HCl.
Laboratory supplies
1. Culture bottles (Tarsons, catalog number: 20080)
2. Pots (Garden Aids, India, catalog number: AP-3)
3. Micropipettes (Eppendorf Research Plus, catalog number: 35016146026)
4. Petri dishes, 90 mm (Abdos, catalog number: 3165077)
5. 2 mL Eppendorf tubes (Abdos, catalog number: P10203)
6. 50 mL centrifuge tubes (Falcon, catalog number: 546024)
7. Filter paper (Sigma, catalog number: FP/310/AC)
8. Forceps (HiMedia, catalog number: LA821)
9. Cotton (HiMedia, catalog number: LA1018)
10. Parafilm (Tarsons, catalog number: 380020)
11. Red soil (PKM Nursery, Thiruvarur, Tamil Nadu)
12. Soilrite (Keltech Energies Limited, HSN code: 68062000)
13. Perlite (Keltech Energies Limited, HSN code: 27030090)
Equipment
1. Weighing balance (Shimadzu, model: ATX224)
2. Autoclave (Equitron, model: PAD NC01HF28837)
3. Centrifuge (Thermo Fisher, model: SORVALST16R)
4. Orbital shaker (C-Being Scientific Inc. model: BSI-3C)
5. Laminar air flow (Lab field, model: LFAS-110)
6. Plant growth chamber (SRL, model: PGC- 11)
7. UV spectrophotometer (Shimadzu, model: UV-1800)
Software and datasets
1. UV probe 2.0
Procedure
文章信息
稿件历史记录
提交日期: Oct 16, 2024
接收日期: Feb 4, 2025
在线发布日期: Feb 26, 2025
出版日期: Mar 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Somna, G., Sangwan, S., Baskar, C., Bakka, K. and Challabathula, D. (2025). Protocol for Inoculation of PGPR Staphylococcus sciuri to Seeds and Seedlings of Rice and Tomato Plants for Increased Root and Shoot Growth. Bio-protocol 15(6): e5241. DOI: 10.21769/BioProtoc.5241.
分类
植物科学 > 植物生理学 > 植物生长
生物科学 > 生物技术 > 微生物技术
植物科学 > 植物免疫 > 宿主-细菌相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











