- EN - English
- CN - 中文
Quantification of Total Free Radicals in Drosophila Using a Fluorescence-Based Biochemical Assay
基于荧光生化检测法的果蝇总自由基定量分析
(*contributed equally to this work, § Technical contact) 发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5238 浏览次数: 1582
评审: Komuraiah MyakalaMinal EngavaleAnonymous reviewer(s)

相关实验方案

使用荧光酶标仪连续测量受细菌感染的骨髓源性巨噬细胞中活性氧的生成
Natascha Brigo [...] Christa Pfeifhofer-Obermair
2023年02月05日 2465 阅读

基于氧化生物标志物评估水生无脊椎动物亚致死声学胁迫的可重复实验方法
Francesca Maria Mitton [...] María Paz Sal Moyano
2026年01月20日 177 阅读
Abstract
Free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), induce oxidative stress. This stress plays crucial roles in cellular signaling, stress response, and disease progression, making the quantification of free radicals essential for understanding oxidative stress mechanisms. Here, we present a high-throughput fluorescence-based protocol for measuring the presence of total free radicals, including ROS and RNS, in the whole adult Drosophila melanogaster (fruit fly). The protocol involves homogenizing whole adult flies in PBS and treating only the supernatant of the lysate with dichlorodihydrofluorescein-DiOxyQ (DCFH-DiOxyQ), which then converts into a fluorescent molecule, dichlorofluorescein (DCF), upon reacting with free radicals. The level of fluorescence is directly proportional to the amount of free radicals present in the sample. This protocol offers simplicity, scalability, and adaptability, making it ideal for studying oxidative stress in the model organism Drosophila and its different tissues under different dietary regimes, environmental stresses, genetic mutations, or pharmacological treatments. It is to be noted that the protocol uses a kit from Abcam, which has been used to measure free radicals in mice, rats, human blood, and cell lines. It can also be applied to biofluids, culture supernatants, and cell lysates, making it suitable for a wide range of sample types beyond whole organisms or tissues. However, due to our research focus and expertise, here we describe a detailed protocol to measure free radicals responsible for inducing oxidative stress only in fruit flies.
Key features
• Quantifies total free radicals including ROS and RNS levels in adult Drosophila melanogaster using a fluorescence-based approach for oxidative stress studies.
• Suitable for high-throughput analysis with a 96-well black plate format, simultaneously enabling efficient handling of multiple samples and standards.
• Adaptable to different experimental conditions, including diverse ROS-inducing treatments and mutations in Drosophila.
• Offers detailed instructions for reagent preparation, sample homogenization, fluorescence measurement, normalization, and statistical analysis of data to ensure reproducibility and accuracy across research settings.
Keywords: Free radicals (自由基)Graphical overview
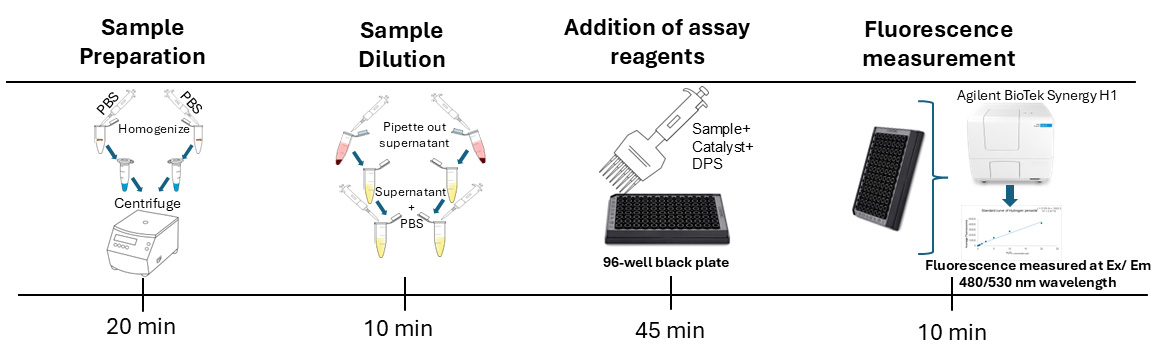
Background
Free radicals, including reactive oxygen species (ROS) and reactive nitrogen species (RNS), are crucial players in cellular homeostasis [1]. Their imbalance contributes to numerous pathological conditions [2], aging [3], cancer [4], and neurodegenerative diseases [4]. ROS and RNS are byproducts of normal metabolic processes [5], primarily from mitochondrial respiration [6]. However, excessive ROS levels or diminished antioxidant defenses cause oxidative stress, which damages proteins, lipids, and nucleic acids [7]. This disruption is observed in several diseases, including Alzheimer's disease [8], Parkinson's disease [9], and various cancers [10]. Research in model organisms like Drosophila melanogaster has greatly advanced our understanding of ROS-related pathophysiology [11].
Several existing methodologies are employed to measure ROS, including chemiluminescence [12], electron spin resonance spectroscopy [13], and flow cytometry [14]. These techniques are highly sensitive but very complex and often require advanced instrumentation, expensive reagents, and specific expertise. Furthermore, they may not be readily adaptable to all experimental designs or accessible to all laboratories. Thus, the need for a simple, scalable, and cost-effective alternative method remains a critical challenge in oxidative stress research.
For our assay, we used three biological replicates, each with two technical replicates following the common practice in biochemical assays using fruit flies [15–17]. Our protocol employs a fluorescence-based approach for quantifying free radicals (ROS and RNS) in Drosophila. This method involves preparing samples by homogenizing whole adult flies, preparing hydrogen peroxide standards, and treating the samples and standards with a non-fluorescent dye, dichlorodihydrofluorescein diacetate (DCFH-DA), which generates the fluorescent product DCF upon reacting with free radicals such as ROS and RNS in the presence of a catalyst (ab238535) [18,19]. Fluorescence is measured using excitation/emission wavelengths of 480/530 nm using a plate reader (Figure 1). The fluorescence of each sample is used to calculate the concentration of free radicals in the sample by using the formula generated in the standard curve analysis. We use the Bradford assay to measure the protein concentration of each sample and normalize the data by dividing the free radical concentration by the protein concentration of the corresponding sample. This is a common practice for biochemical assays [15–17] to calculate the relative amount of free radicals per unit of protein. Normalization of data helps to avoid false differences in fluorescence levels between two samples due to different dilutions as a result of human and technical errors.
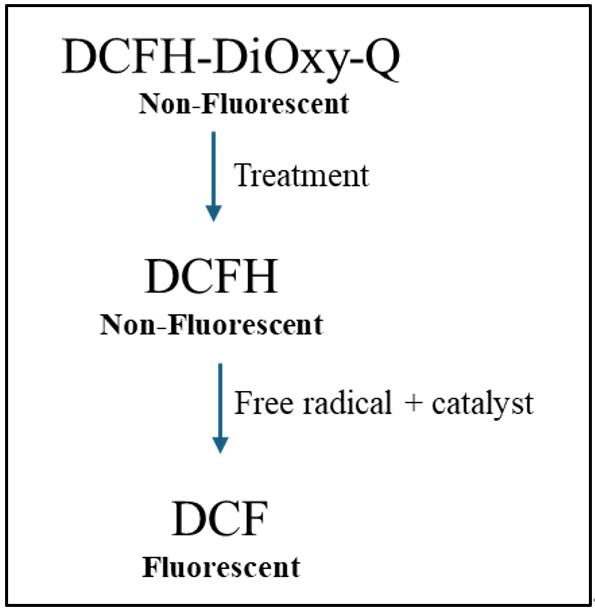
Figure 1. Mechanism of in vitro reactive oxygen species (ROS)/reactive nitrogen species (RNS) assay. Dichlorodihydrofluorescein-DiOxyQ (DCFH-DiOxyQ), a non-fluorescent quenched dye, is treated first with a priming solution to form DCFH-DiOxy. Treatment with a stabilization solution converts it into DCFH, which remains non-fluorescent. In the presence of ROS/RNS and a catalyst (such as an esterase), DCFH is oxidized to fluorescent dichlorofluorescein (DCF), allowing ROS/RNS detection [19].
A significant advantage of this protocol is its accessibility and scalability, requiring only standard laboratory equipment and reagents. It allows researchers to investigate oxidative stress in various biological contexts, including dietary intervention, the impact of genetic mutations, environmental stressors, and therapeutic interventions in Drosophila. Although this protocol does not quantify specific types of free radicals, it provides a reliable measure of overall ROS and RNS levels in flies, which is often sufficient for many experimental objectives. With its simplicity and versatility, this protocol represents a significant step forward in the field of oxidative stress research in fruit flies.
Materials and reagents
Biological materials
1. Drosophila melanogaster (collected from Bloomington Drosophila stock center)
Reagents
1. DCF ROS/RNS assay kit (Abcam, catalog number: ab238535); contains enough reagents to perform 96 assays (Table 1)
Table 1. Storage conditions, concentrations, and volume of individual components from the kit
| Item | Stock concentration | Volume | Storage conditions |
| Priming reagent | 1× | 250 μL | 4 °C |
| Stabilization solution | 10× | 1.5 mL | 4 °C |
| Catalyst | 250× | 20 μL | 4 °C |
| DCF-DiOxyQ | 40× | 50 μL | -20 °C |
| Hydrogen peroxide (H2O2) | 4,400× | 100 μL | 4 °C |
2. Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7906-50G)
3. Bradford reagent (Thermo Scientific, catalog number: 23238)
4. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S7653)
5. Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333)
6. Disodium phosphate (Na2HPO4) (Apex, catalog number: 20-147)
7. Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
Solutions
1. 1× stabilization solution (see Recipes)
2. 1× catalyst (see Recipes)
3. 1× DPS (see Recipes)
4. Hydrogen peroxide standard (see Recipes)
5. BSA standard (see Recipes)
6. 1× phosphate buffer saline (see Recipes)
Recipes
Note: Recipes below are for 50 reactions (one reaction per well of a 96-well plate). Prepare a little extra volume than the exact volume required to make up for any pipetting error.
1. 1× stabilization solution (5.5 mL)
Mix 550 μL of 10× stabilization solution (from the assay kit) with 4,950 μL of deionized water to make 1× solution. Vortex and store at 4 °C until use.
2. 1× catalyst (2.75 mL)
Mix 11 μL of 250× catalyst (from the assay kit) with 2,739 μL of 1× PBS (Recipe 6) to prepare 1× catalyst solution. Vortex and use immediately.
3. 1× DPS (2.75 mL)
Mix 27.5 μL of DCF-DiOxyQ with 110 μL of priming reagent. Vortex and incubate at room temperature for 30 min. Then, add this mixture to 5362.5 μL of 1× stabilization solution (Recipe 1). Cover the tube in aluminum foil. Store on ice for immediate use. Any excess can be stored at -20 °C for up to a week for future assays.
4. Hydrogen peroxide standards
Critical: Freshly prepared sets of standards are made in tubes wrapped with aluminum foil. Prepare a 2 mM H2O2 stock solution by mixing 1 μL of 4,400× H2O2 with 4.4 mL of deionized water. This stock solution is used to prepare standards. See Table 2 for H2O2 standard preparations with 1× PBS (Recipe 6).
Table 2. Hydrogen peroxide standards
| Standard # | 2 mM H2O2 Standard (μL) | 1× PBS (μL) | H2O2 conc. (μM) |
| 1 | 10 | 990 | 20 |
| 2 | 500 μL of standard 1 | 500 | 10 |
| 3 | 500 μL of standard 2 | 500 | 5 |
| 4 | 500 μL of standard 3 | 500 | 2.5 |
| 5 | 500 μL of standard 4 | 500 | 1.25 |
| 6 | 500 μL of standard 5 | 500 | 0.625 |
| 7 | 500 μL of standard 6 | 500 | 0.313 |
| 8 | 0 | 1,000 | 0 (blank) |
Note: In this protocol, the standard curve for hydrogen peroxide has been adjusted from the original range of 0.039 μM to 0.313 μM, as specified in the protocol for the ab238535 kit (Abcam). This modification is based on the observation that the lower concentration range (0.039 μM) is not necessary for the sensitivity and detection limits of the assay under the conditions used in this study. In our sample, the free radical concentration equivalent to H2O2 concentration in the standard curve fell between 10 and 20 μM. However, researchers can use lower concentrations of H2O2 in the standard curve as suggested by the Abcam protocol if they use a lower number of flies or a higher dilution of fly samples.
5. BSA standards
Prepare 2 mg/mL BSA stock solution by combining 40 μL of BSA stock (50 mg/mL) with 960 μL of 1× PBS (Recipe 6), yielding a total volume of 1,000 μL. Then, prepare a series of standard dilutions from the 2 mg/mL BSA stock solution with varying concentrations in cooled 1× PBS as shown in Table 3.
Table 3. BSA standards
| BSA | Concentration (mg/mL) | Volume from previous standard (μL) | 1× PBS (μL) | Remaining total volume (μL) |
| Blank | 0 | 0 | 25 | 25 |
| Standard 1 | 0.125 | 25 of 0.25 mg/mL | 25 | 50 |
| Standard 2 | 0.25 | 25 of 0.5 mg/mL | 25 | 25 |
| Standard 3 | 0.50 | 25 of 1 mg/mL | 25 | 25 |
| Standard 4 | 1.00 | 25 of 2 mg/mL | 25 | 25 |
| Standard 5 | 2.00 | 50 | 0 | 25 |
6. 1× phosphate buffer saline (PBS)
a. Begin by making a 10× PBS solution, placing 250 mL of distilled water in a 500 mL container.
b. Add the following reagents to the distilled water:
• 80.06 g of NaCl (1.37 M)
• 2.01 g of KCl (27 mM)
• 26.8 g of Na2HPO4 (100 mM)
• 2.45 g of KH2PO4 (18 mM)
c. Adjust the pH of the solution to 7.4 using a pH meter.
d. Add distilled water to bring the total volume up to 500 mL.
e. Aliquot prepared 10× PBS into five bottles, each containing approximately 100 mL of PBS. Sterilize by autoclaving for 30 min at 121 °C.
f. Dilute the 10× PBS stock solution 1:10 with distilled water. For example, mix 50 mL of 10× PBS with 450 mL of distilled water to make 500 mL of 1× PBS.
Laboratory supplies
Note: Similar lab supplies from other companies will work as well.
1. 96-well transparent plate (Genesee Scientific, catalog number: 25-109)
2. 96-well black plate (Thermo Scientific, catalog number: 165305)
3. PolyPestle (Genesee Scientific, catalog number: 25-280)
4. Microcentrifuge tube (clear polypropylene 1.7 mL tube) (Olympus, catalog number: 24-282)
5. Single (Sartorius brand, 1–10, 10–100, 20–200, 100–1,000 μL) pipette and multi-channel pipettes (Poseidon 8 Channel P300 Pipettor, 20–300 μL)
6. Sterile tips (Olympus brand, 10, 100, 200, and 1,000 μL tips)
7. Pasteur pipette (VWR, catalog number: 14672-200)
Equipment
1. Agilent BioTek Synergy H1; filter set: excitation at 480 nm and emission at 530 nm
Note: Any other plate reader that can measure the above fluorescence wavelengths can be used.
2. Microcentrifuge (Hermle, catalog number: Z216-MK)
3. Vortex (Benchmark, catalog number: BV1000)
4. Mini centrifuge (Genesee Scientific, catalog number: 27-523)
5. Autoclave (Priorclave, catalog number: 150-320L)
6. -80 °C freezer (Eppendorf, catalog number: EPP: F740540015)
7. -20 °C freezer (VWR, catalog number: 76580-128)
8. pH meter (Thermo Scientific, model: Orion Star A211)
Software and datasets
1. GraphPad Prism (https://graphpad-prism.software.informer.com/7.0/)
2. Gen5 3.14 ( https://www.agilent.com/en/support/biotek-software-releases)
3. Microsoft Excel
Procedure
文章信息
稿件历史记录
提交日期: Nov 27, 2024
接收日期: Jan 26, 2025
在线发布日期: Feb 26, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Arzoo, S. H., Tasmin, R. and Banerjee, S. J. (2025). Quantification of Total Free Radicals in Drosophila Using a Fluorescence-Based Biochemical Assay. Bio-protocol 15(5): e5238. DOI: 10.21769/BioProtoc.5238.
分类
生物化学 > 其它化合物 > 活性氧
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









