- EN - English
- CN - 中文
Integrated Co-extraction Protocol for Transcriptomic and 1H NMR Metabolomic Analysis of Multi-species Biofilms
多物种生物膜的转录组与¹H NMR代谢组联合提取方案
发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5237 浏览次数: 1967
评审: Emilia KrypotouBassam A. ElgamoudiAnonymous reviewer(s)
Abstract
Capturing produced, consumed, or exchanged metabolites (metabolomics) and the result of gene expression (transcriptomics) require the extraction of metabolites and RNA. Multi-omics approaches and, notably, the combination of metabolomics and transcriptomic analyses are required for understanding the functional changes and adaptation of microorganisms to different physico-chemical and environmental conditions. A protocol was developed to extract total RNA and metabolites from less than 6 mg of a kind of phototrophic biofilm: oxygenic photogranules. These granules are aggregates of several hundred micrometers up to several millimeters. They harbor heterotrophic bacteria and phototrophs. After a common step for cell disruption by bead-beating, a part of the volume was recovered for RNA extraction, and the other half was used for the methanol- and dichloromethane-based extraction of metabolites. The solvents enabled the separation of two phases (aqueous and lipid) containing hydrophilic and lipophilic metabolites, respectively. The 1H nuclear magnetic resonance (NMR) analysis of these extracts produced spectra that contained over a hundred signals with a signal-to-noise ratio higher than 10. The quality of the spectra enabled the identification of dozens of metabolites per sample. Total RNA was purified using a commercially available kit, yielding sufficient concentration and quality for metatranscriptomic analysis. This novel method enables the co-extraction of RNA and metabolites from the same sample, as opposed to the parallel extraction from two samples. Using the same sample for both extractions is particularly advantageous when working with inherently heterogeneous complex biofilm. In heterogeneous systems, differences between samples may be substantial. The co-extraction will enable a holistic analysis of the metabolomics and metatranscriptomics data generated, minimizing experimental biases, including technical variations and, notably, biological variability. As a result, it will ensure more robust multi-omics analyses, particularly by improving the correlation between metabolic changes and transcript modifications.
Key features
• Co-extraction of metabolites and total RNA from 6 mg of dry biomass of phototrophic biofilms, notably oxygenic photogranules.
• Biphasic metabolome extraction for the characterization of hydrophilic and lipophilic metabolites using 1H NMR.
• Total RNA extraction with sufficient quality and quantity for analysis of (meta)transcriptome.
Keywords: Biofilm (生物膜)Graphical overview
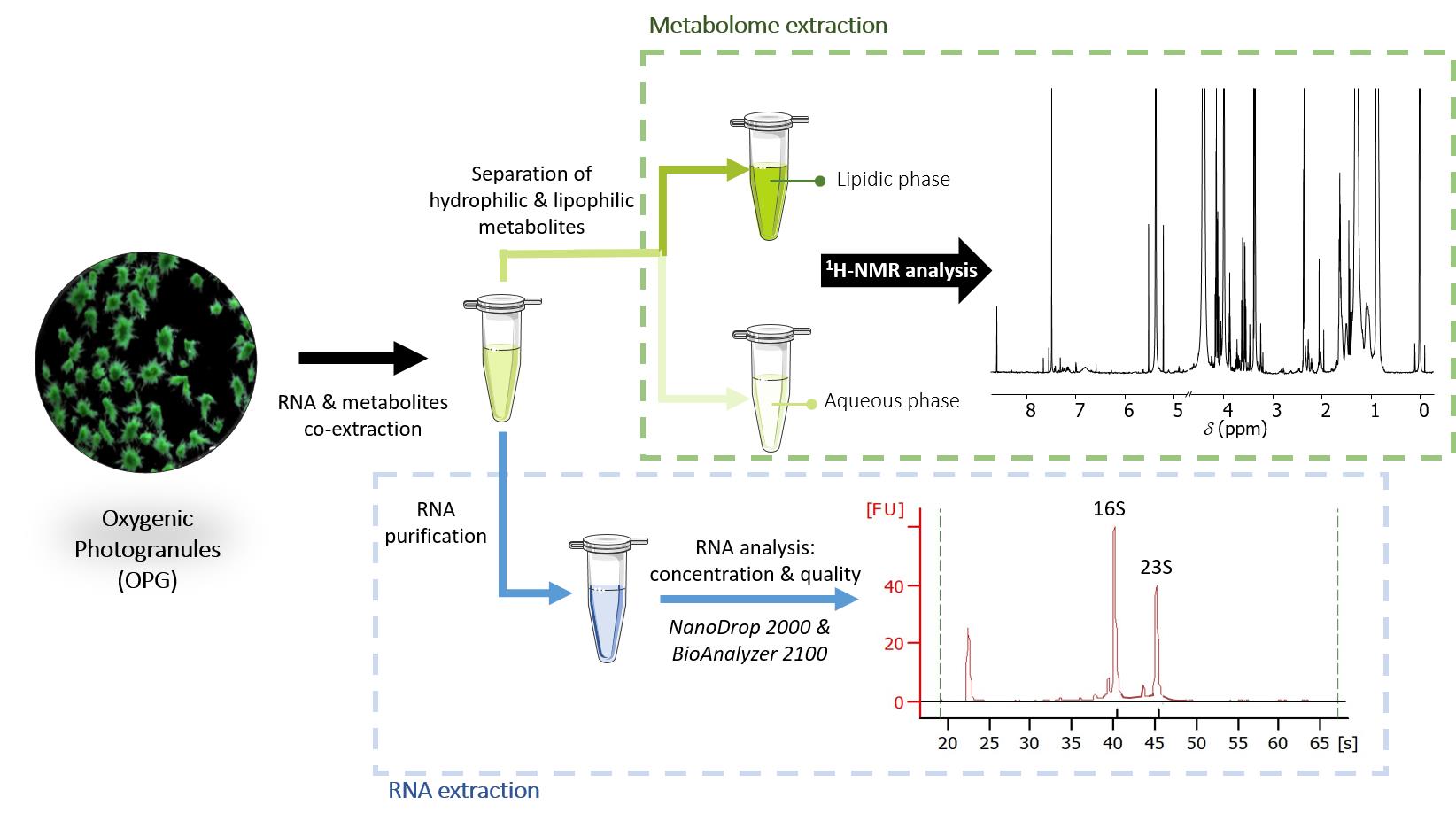
Background
In contrast to suspended individual cells, biofilms are spatialized microbial structures composed of diverse interacting microbial populations. Gradients created by energy and mass transfer as well as the production and consumption of metabolites in the biofilm matrix generate a wide range of physiological states of the resident microbial community. The biofilm environment implies genetic and phenotypic heterogeneity, with distinct metabolic pathways and varied responses to stress and environmental conditions along the gradients. These sources of heterogeneity affect functional and metabolic responses in space and time [1,2].
Elucidating the physiologies of biofilm-associated microorganisms by capturing metabolite production and gene expression is key to deciphering how multi-species biofilms adapt to changing environments as a community. Various methods are currently available to study the dynamics of biofilms, including metabolomics [3–5] and transcriptomic [6,7] approaches. Multi-omics approaches, combining metatranscriptomics and metabolomics through separate metabolome and RNA extraction procedures, have been applied to the study of some biofilms [8–10]. However, to the best of our knowledge, a protocol that enables the simultaneous extraction of both metabolites and RNA from biofilms has not yet been described.
Metabolomics focuses on the detection of metabolites (for example, produced during biofilm formation) and characterizes the state of the metabolome at a given time. In this way, metabolomics provides a view of the general physiological state of the biofilm community in a given condition. Metabolite profiles are functional signatures that can indicate an actual phenotype [5]. Nuclear magnetic resonance (NMR) spectroscopy is increasingly used in metabolomics and biofilm characterization due to the simplicity of measurements compared with other methods such as mass spectrometry. Compared to mass spectrometry, NMR is less sensitive, focuses on the major changes in metabolic pathways, and is not sufficient to profile all the metabolites and identify all functional changes [11]. However, NMR offers numerous advantages as it generally includes a simplified sample pre-treatment and allows immediate (semi) quantification of metabolites and robust metabolite assignment [12]. It is independent of ionization efficiency and can detect a wide range of metabolites, including those that ionize poorly in mass spectrometry, such as sugars, organic acids, and some lipids usually found in biofilms. Thus, this method is particularly adapted for characterizing and identifying compositional changes in biofilms.
Metatranscriptomic analysis enables the study of the total mRNA transcripts and reveals the global gene expression profiles and changes. It can give insights into the functional components activated or inhibited during biofilm growth or decay. It can also be used to monitor biofilm dynamics in response to changes in physico-chemical or environmental conditions [13].
Currently, most omics studies are conducted separately, resulting in a partial picture of the state of the biological system. If conducted together, they generally result in distinct extraction procedures [8–10]. The integration of metabolomic and metatranscriptomic approaches offers the potential to uncover novel correlations between metabolites and transcripts. However, in biofilms, their inherent structural, microbiological, and functional heterogeneity amplifies the variability of results when multiple extractions are performed. To minimize experimental biases, including technical variations and biological variability, a protocol that allows the simultaneous extraction of RNA and metabolites is essential. Co-extraction enhances the robustness of the results by ensuring that metabolic and transcriptomic changes can be directly correlated within the same sample, providing a more reliable and comprehensive understanding of biofilm dynamics.
In this study, oxygenic photogranules (OPGs) were used as a model biofilm system to develop a co-extraction protocol for metabolites and RNA, enabling NMR-based metabolomics and metatranscriptomic analyses. OPGs are three-dimensional aggregates with spatial and compositional (microbial communities and metabolites) heterogeneity. They contain a syntrophic community composed of heterotrophic and phototrophic microorganisms (eukaryotic algae and cyanobacteria) [14]. They are obtained in controlled conditions from activated sludge and are currently under study for wastewater treatment [15].
Materials and reagents
Biological materials
Metabolites and RNA were co-extracted from oxygenic photogranules (OPGs) (Figure 1). Photogranules are microbial aggregates in the size range of several hundreds of micrometers up to several millimeters. They harbor a microbial community primarily composed of a phototrophic population, often dominated by one or two filamentous cyanobacteria of the order Oscillatoriales and a taxonomically and functionally diverse population of heterotrophic bacteria. Photogranules were produced using previously described protocols [14,15]. Briefly, OPGs were grown in an open sequencing batch reactor operated at an average temperature of 25 °C with overhead stirring of 110 rpm. LED panels provided white light at 4,000 K, yielding 96 μmol/m2/s photosynthetically active radiation at the outside of the vertical surfaces of the reactor. The reactor had a working volume of 4 L. The sequencing batch cycle had a duration of 3 h. Two liters of bulk phase were replaced in every cycle with a synthetic medium at a carbon concentration of 33 mg/L as chemical oxygen demand, resulting in a hydraulic retention time of 6 h. The synthetic medium contained 0.5 mM sodium acetate, 6 μM KH2PO4, 5 μM Na2HPO4, 0.13 mM (NH4)2SO4, 1.1 μM EDTA·2H2O, 0.9 nM Na2MoO4·2H2O, 6 nM FeSO4·7H2O, 0.042 μM H3BO3, 3 nM ZnSO4·7H2O, 2 nM MnCl2, 7 nM CoCl2·6H2O, 0.01 μM CuSO4·5H2O, 0.2 μM FeCl3, and 0.9 nM NiCl2·6H2O.
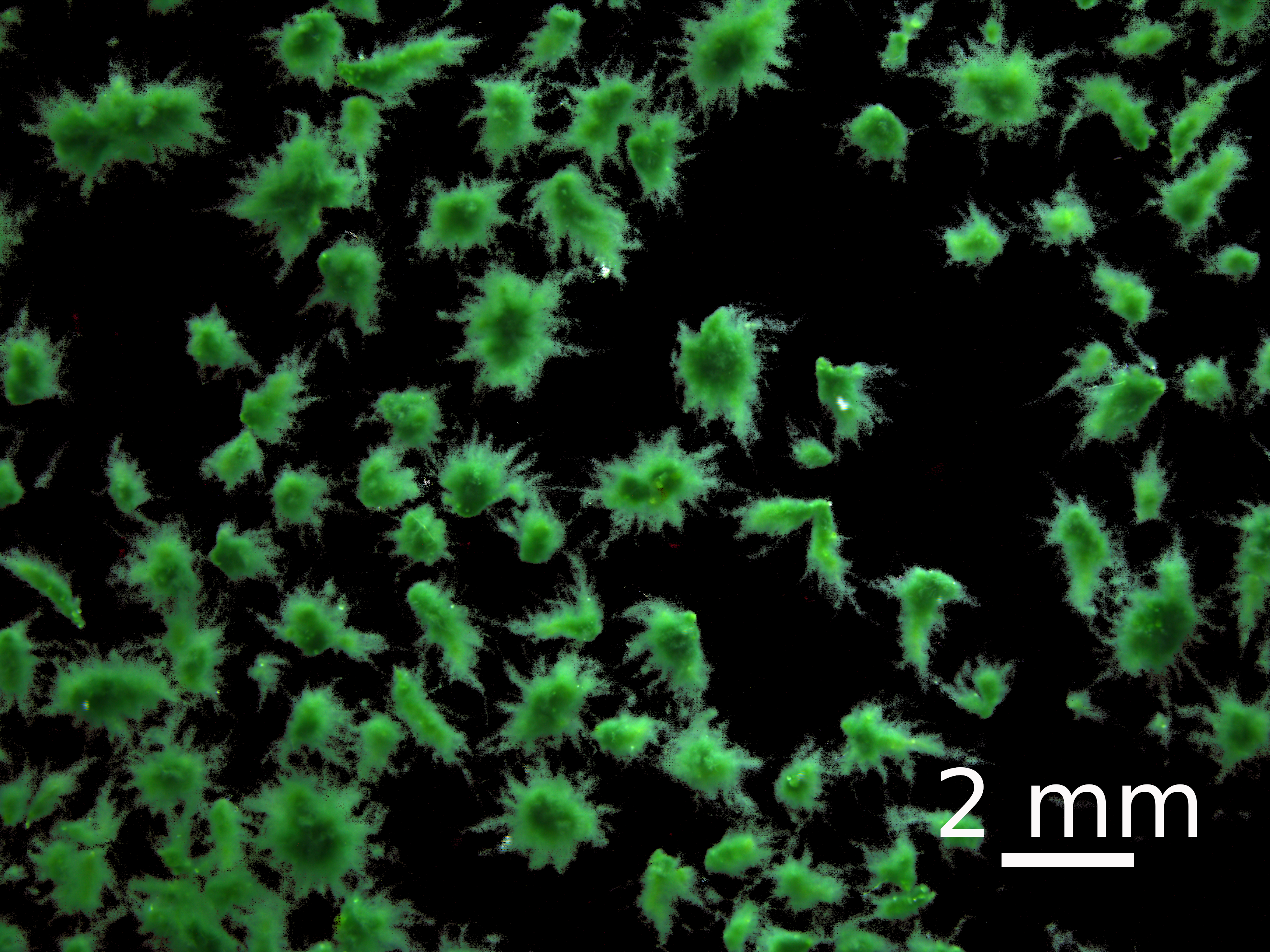
Figure 1. Stereomicroscopic image of photogranules obtained after the sieving step
Reagents
1. Ethanol absolute (Fisher, catalog number: 10048291)
2. Methanol (Fisher, catalog number: 10499560)
3. Dichloromethane (Fisher, catalog number: 10626642)
4. Disodium hydrogen phosphate anhydrous (Na2HPO4) (Sigma-Aldrich, catalog number: 71640)
5. Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P5655)
6. 3-(Trimethylsilyl)propionic acid-d4 sodium salt (TSP) (Sigma-Aldrich, catalog number: 269913)
7. Deuterium oxide (D2O) (Sigma-Aldrich, catalog number: 151882)
8. Methanol-d4 (CD3OD) (Sigma-Aldrich, catalog number: 151947)
9. Chloroform-d (CDCl3) + 0.03% trimethylsilane (TMS) (Sigma-Aldrich, catalog number: 343803)
10. RNeasy Mini kit (Qiagen, catalog number: 74104)
11. Qubit 1× dsDNA HS Assay kit (Thermo Fisher Scientific, InvitrogenTM, catalog number: Q33230)
12. DNA-freeTM DNA Removal kit (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM1906)
12. RNaseZap (Thermo Fisher Scientific, InvitrogenTM, catalog number: AM9780)
14. RNA 6000 Nano kit (Agilent, catalog number: 5067-11511)
Solutions
1. 70% ethanol solution (see Recipes)
2. Buffer phosphate solution (see Recipes)
Recipes
1. 70% ethanol solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol absolute | 70% | 700 mL |
| MilliQ water (H2O) | n/a | 300 mL |
| Total | n/a | 1,000 mL |
2. Buffer phosphate solution
Weigh all components mentioned below and dissolve in 100 mL of deuterated water. Measure and adjust the pH to 7.0 with 5 M HCl.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4 | 200 mM | 2.885 g |
| KH2PO4 | 40 mM | 595 mg |
| TSP | 0.2 mM | 3.45 mg |
| D2O | n/a | 100 mL |
| Total | n/a | 100 mL |
Laboratory supplies
A. Sampling
1. Lysing matrix E (MP Biomedicals, catalog number: 1169140-CF)
2. Sieves (200 mm DIA × 50 mm ISO 3310–1); 0.6 mm (Retsch, catalog number: 60131000600, 23208274) and 1.0 mm (Fisher Scientific, catalog number: 10536222, 7156588)
3. Liquid nitrogen
4. Dry ice
B. Co-extractions
1. QIAshredder (Qiagen, catalog number: 79654)
3. 1.5 mL microtubes (e.g., Eppendorf, catalog number: 0030125150)
4. Filter tips 1,250, 200, and 20 μL (e.g., Clearline, catalog numbers: 713119, 713117, 713115)
5. 2, 10, 20, 200, and 1,000 μL pipettes (e.g., Clearline or Gilson)
C. NMR analysis
1. 1.75 mL Eppendorf tubes (Dutscher, Greiners, catalog number: 2710757)
2. Pipette tips 1,250 and 200 μL (Dutscher, Clearline, catalog number: 713113, 713111)
3. Pasteur pipette (Dutscher, catalog number: 2517260)
4. NMR tubes 3 mm including caps (Bruker, catalog number: Z112272)
5. Cap sealing balls (Bruker, catalog number: Z147554)
Equipment
1. Laboratory fume hood (e.g., Waldner, model: MC6 1500 mm)
2. FastPrep-24TM 5G bead beating grinder and lysis system (MP Biomedicals, catalog number: 116005500)
3. Microcentrifuge (e.g., Eppendorf, model: 5425)
4. Refrigerated centrifuge (e.g., Sartorius, model Centrisart A-14C; Mikkro 220R, Dutscher, catalog number: 472328)
5. Multivortex Multireax (Heidolph, Dutscher, catalog number: 498446)
6. Bioanalyzer 2100 (Agilent, catalog number: G2939BA)
7. Nanodrop ND2000 (Thermo Scientific, catalog number: ND-2000)
8. Qubit 4 fluorometer (Thermo Scientific, catalog number: Q33238)
9. -20 °C freezer
10. -80 °C freezer
11. pH meter HI 3221 (Hanna Instruments, Dutscher, catalog number: 054585)
12. SpeedVac SPD 300 DDA (Dutscher, Fischer, catalog numbers: 22880, 228324, 228731)
13. Sample preparation robot (Tecan, model: Fluent 780)
Software and datasets
1. TopSpin (Bruker Biospin, Germany, V3.6.4, February 2023)
2. R software (https://cran.r-project.org/, V4.2.2, November 2022)
3. ASICS R package (V2.20.1)
4. 2100 expert software (Agilent, V B.02.10.764, July 2018)
5. Nanodrop 2000 software (V1.6.198, August 2014)
Procedure
文章信息
稿件历史记录
提交日期: Dec 6, 2024
接收日期: Feb 4, 2025
在线发布日期: Feb 26, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Séguéla, A., Della-Negra, O., Gautier, R., Hamelin, J., Milferstedt, K., Servien, R., Teste, M. and Canlet, C. (2025). Integrated Co-extraction Protocol for Transcriptomic and 1H NMR Metabolomic Analysis of Multi-species Biofilms. Bio-protocol 15(5): e5237. DOI: 10.21769/BioProtoc.5237.
分类
微生物学 > 微生物生物膜
系统生物学 > 转录组学
系统生物学 > 代谢组学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












