- EN - English
- CN - 中文
Rapid and Efficient Isolation of Total RNA-Bound Proteomes by Liquid Emulsion–Assisted Purification of RNA-Bound Protein (LEAP-RBP)
液体乳状液辅助纯化RNA结合蛋白(LEAP-RBP)快速高效分离总RNA结合蛋白质组
发布: 2024年07月20日第14卷第14期 DOI: 10.21769/BioProtoc.5236 浏览次数: 2593
评审: Dipak Kumar PoriaThirupugal GovindarajanAnonymous reviewer(s)

相关实验方案

从大肠杆菌中大规模纯化III型毒素-抗毒素核糖蛋白复合物及其成分,用于生物物理学研究
Parthasarathy Manikandan [...] Mahavir Singh
2023年07月05日 2174 阅读

用于Poly(A)纯化mRNA中N6-甲基腺苷定量的改良型m6A-ELISA方法
Wei Yee Chan [...] Folkert J. Van Werven
2025年06月20日 3007 阅读
Abstract
The critical roles of RNA-binding proteins (RBPs) in all aspects of RNA biology fostered the development of methods utilizing ultraviolet (UV) crosslinking and method-specific RNA enrichment steps for proteome-wide identification and assessment of RBP function. Despite the substantial contributions of these UV-based RNA-centric methods to our understanding of RNA–protein interaction networks, their utility is constrained by biases in RBP recovery and significant noise contributions, which can confound meaningful interpretation. To overcome these issues, we recently developed a method termed Liquid Emulsion–Assisted Purification of RNA-Bound Protein (LEAP-RBP) and introduced quantitative signal-to-noise (S:N)-based metrics for the proteome-wide identification of RNA interactomes and accurate assessment of global RBP occupancy dynamics. Compared to existing methodologies, LEAP-RBP provides significant advantages in speed, cost, efficiency, and selectivity for RNA-bound proteins. In this work, we provide a step-by-step guide for the successful application of the LEAP-RBP method for both small- and large-scale investigations of RNA-bound proteomes.
Key features
• Unbiased and efficient isolation of total RNA-bound protein, RNA, and protein from biological samples.
• Cost-effective identification of proteome-wide RNA interactomes and validation of direct RNA-binding protein functionality.
• Robust and accurate assessment of context- and/or condition-dependent RBP occupancy state dynamics.
Keywords: LEAP-RBP (LEAP-RBP)Graphical overview
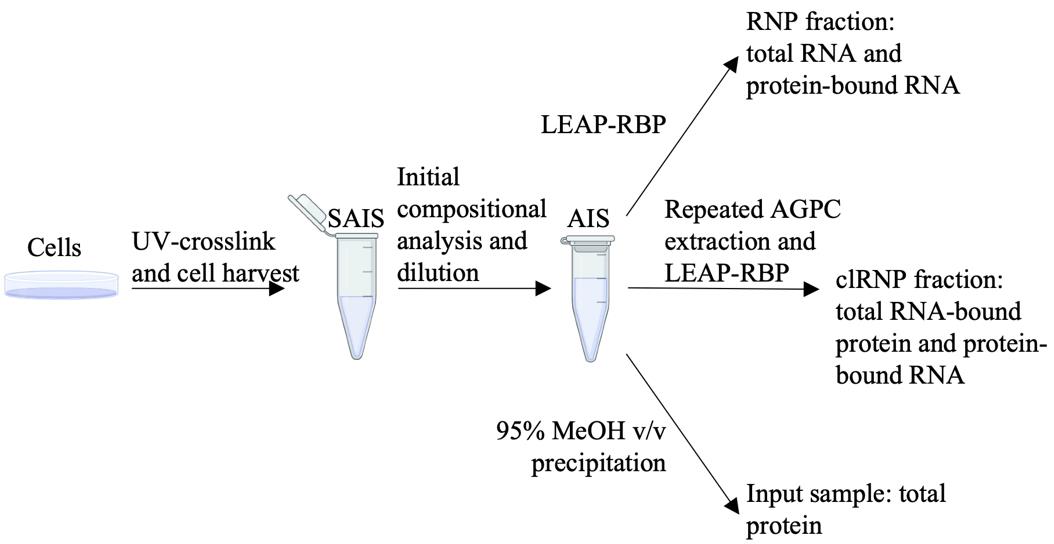
Background
With the growing interest in the identification and study of RNA interactomes, an expanding toolkit of methods to capture RNA-protein complexes has appeared [1–9]. The most widely utilized methods employ UV crosslinking and different RNA-enrichment or organic phase–separation conditions for selective isolation of RNA–protein complexes and can be categorized as UV-based RNA-centric methods, or RNA-centric methods for short [10–17]. When paired with downstream quantitative mass spectrometry, these methods address the need for proteome-wide identification of candidate RNA-binding proteins (RBPs). We recently reported an RNA-centric method termed Liquid Emulsion–Assisted Purification of RNA-Bound Protein (LEAP-RBP), which can be distinguished from existing methods by its high selectivity for protein–RNA complexes via the use of a lithium-supplemented heterogeneous solvent system [18]. Although the precise biochemical/biophysical mechanism for the high selectivity of LEAP-RBP for protein–RNA adducts remains conjecture, we postulate that lithium complexation with the RNA component of protein–RNA adducts promotes selective precipitation, consistent with the established, but not well understood, capacity for lithium to precipitate RNA [19]. Importantly, the high selectivity of the LEAP-RBP method for protein–RNA complexes enables signal-to-noise (S:N)-based metrics for quantitative evaluation of protein–RNA interactions. Combined, LEAP-RBP addresses limitations in existing methodologies, which include biases in RBP recovery due to the inclusion of stringent denaturing washes, confounding levels of experimental noise (i.e., free protein recovery in the RBP fraction), and a paucity of quantitative metrics for assessing RNA binding function [18]. The utility of the LEAP-RBP method has been further elaborated in the identification of RBPs undergoing a condition-dependent change in RNA occupancy following short-term inhibition of translation initiation [18]. These included both RBPs with well-established roles in translation-related processes such as UPF1 and RPS3 and proteins with previously unknown roles in RNA regulation such as ABCF3 [18]. In summary, the high protein–RNA complex specificity and efficiency of the LEAP-RBP method supports rapid and inexpensive orthogonal validation of RBP occupancy state changes and RNA-binding function of candidate RBPs, using basic laboratory techniques such as SDS-PAGE RNase-dependent Assay (SRA). The simplicity and broad applicability of the LEAP-RBP method position it as a valuable tool for low- and high-throughput identification of RNA-binding proteins and rigorous assessment of RBP occupancy state change dynamics.
Materials and reagents
2-mercaptoethanol (Sigma-Aldrich, catalog number: M7522)
Acidic phenol pH 4.5 (VWR, catalog number: 0981)
Chloroform, ethanol stabilizer (Thermo Fisher Scientific, catalog number: MCX10601)
Isopropanol (VWR, catalog number: BDH1133)
Methanol (MeOH) (VWR, catalog number: BDH1135)
Sodium citrate (VWR, catalog number: BDH9288)
Citric acid (Sigma-Aldrich, catalog number: C0759)
EDTA disodium salt dihydrate (VWR, catalog number: 0105)
N-lauroylsarcosine sodium salt (Sigma-Aldrich, catalog number: L9150)
Guanidine thiocyanate (Chem-Impex, catalog number: 00522)
Diethyl pyrocarbonate (DEPC) (RPI, catalog number: D43060)
Hydrochloric acid (Ricca Chemical, catalog number: RABH0010)
TRIS base (VWR, catalog number: 97062)
LiDS (Thermo Fisher Scientific, catalog number: J32816)
LiCl (VWR, catalog number: ALFA10515; Sigma-Aldrich, catalog number: 213233)
Sodium hydroxide (VWR, catalog number: BDH9292)
Turbo DNase kit (Thermo Fisher Scientific, catalog number: AM2238)
Pierce BCA kit (Thermo Fisher Scientific, catalog number: 23225)
Dulbecco’s phosphate-buffered saline (PBS) (Thermo Fisher Scientific, catalog number: 14190144)
Solutions
DEPC-treated ultrapure H2O (see Recipes)
5 M GT (see Recipes)
750 mM sodium citrate pH 7.0 (see Recipes)
10% N-lauroylsarcosine (m/v) (see Recipes)
0.5 M EDTA pH 8.0 (see Recipes)
GT buffer (see Recipes)
AGP (see Recipes)
1.0 M Tris-HCl pH 8.0 (see Recipes)
10% LiDS (m/v) (see Recipes)
1% LiDS TE (see Recipes)
2% LiDS TE (see Recipes)
10 M LiCl (see Recipes)
Precipitation solution (see Recipes)
95% MeOH (see Recipes)
Recipes
DEPC-treated ultrapure H2O (1 L)
Reagent Final concentration Amount or volume Ultrapure H2O n/a 999.0 mL Diethyl pyrocarbonate (DEPC) 0.1% v/v 1 mL Total n/a 1 L Prepare in an airtight glass container. Shake vigorously for 5 s to dissolve DEPC. Incubate overnight at 37 °C and autoclave to decompose unreacted DEPC. Shelf life is undetermined, > 1 year.
5 M GT (0.5 L)
Reagent Final concentration Amount or volume Guanidine thiocyanate 5 M 295.4 g DEPC-treated ultrapure H2O n/a 271 mL Total n/a 0.5 L Add in the order listed. Weigh guanidine thiocyanate and adjust the volume of DEPC-treated ultrapure H2O accordingly. Prepare in a beaker using a magnetic stir bar and hot plate stirrer set to 37 °C. Clarify by filtering twice using Whatman paper or by standing incubation overnight and transferring the clarified portion. Store in an airtight glass container at room temperature (RT). Shelf life is undetermined, > 1 year. Protect from light during all stages of the preparation procedure.
750 mM sodium citrate pH 7.0 (50 mL)
Reagent Final concentration Amount or volume Citric acid anhydrous n/a 0.13 g Sodium citrate dihydrate n/a 10.83 g DEPC-treated ultrapure H2O n/a Adjust volume to 50 mL Total n/a 50 mL Add in the order listed. Prepare in a 50 mL conical tube. Weigh citric acid anhydrous and adjust the amount or volume of other components accordingly. Dissolve by incubating on a rotator at RT. Filter using a syringe equipped with a 0.2 µm cellulose acetate syringe filter. Store at RT. Shelf life is undetermined, > 1 year.
10% N-lauroylsarcosine m/v (10 mL)
Reagent Final concentration Amount or volume N-lauroylsarcosine sodium salt 10% m/v 1 g DEPC-treated ultrapure H2O n/a 9 mL Total n/a 10 mL Add in the order listed. Prepare in a 15 mL conical tube. Weigh N-lauroylsarcosine sodium salt and adjust the volume of DEPC-treated ultrapure H2O accordingly. Dissolve by incubating on a rotator at RT. Store at 4 °C. Shelf life is undetermined, > 6 months.
0.5 M EDTA pH 8.0 (50 mL)
Reagent Final concentration Amount or volume Sodium hydroxide pellets n/a 1.28 g DEPC-treated ultrapure H2O n/a 44.27 mL EDTA disodium salt dihydrate 0.5 M 9.31 g Total n/a 50 mL Add in the order listed. Prepare in a 50 mL conical tube. Weigh sodium hydroxide pellets and adjust the amount or volume of other components accordingly. Dissolve by incubating on a rotator at RT. Filter using a syringe equipped with a 0.2 µm cellulose acetate syringe filter. Store at RT. Shelf life is undetermined, > 1 year.
GT buffer (50 mL)
Reagent Final concentration Amount or volume DEPC-treated ultrapure H2O n/a 5 mL 5 M GT 4 M 40 mL 750 mM sodium citrate pH 7.0 25 mM 1.67 mL 0.5 M EDTA pH 8.0 5 mM 500 µL 10% N-lauroylsarcosine m/v 0.5% m/v 2.5 mL 2-mercaptoethanol 0.7% v/v 350 µL Total n/a 50 mL Add in the order listed. Prepare in a 50 mL conical tube. Can be prepared without N-lauroylsarcosine and 2-mercaptoethanol and stored at RT. Protect from light. Shelf life of GT buffer (−N-lauroylsarcosine m/v and 2-mercaptoethanol) is undetermined, > 1 year. Use within a day after adding of N-lauroylsarcosine and 2-mercaptoethanol or store at -80 °C (shelf life is undetermined, > 1 year). Mix stock solutions before adding.
AGP (10 mL)
Reagent Final concentration Amount GT buffer 66% v/v 6.67 mL Acidic phenol pH 4.5 n/a 3.33 ml Total n/a 10 mL Add in the order listed. Combine reagents in a 15 mL conical tube and store at RT. Protect from light. Use within a day or store at -80 °C (shelf life is undetermined, > 1 year).
1.0 M Tris-HCl pH 8.0 (50 mL)
Reagent Final concentration Amount or volume TRIS base 1.0 M 6.06 g DEPC-treated ultrapure H2O n/a 43.1 mL Hydrochloric acid n/a 2.35 mL Total n/a 50 mL Add in the order listed. Prepare in a 50 mL conical tube. Dissolve by incubating on a rotator at RT. Filter using a syringe equipped with a 0.2 µm cellulose acetate syringe filter. Store at RT. Shelf life is undetermined, > 1 year.
10% LiDS m/v (50 mL)
Reagent Final concentration Amount or volume LiDS 10% m/v 5 g DEPC-treated ultrapure H2O n/a Adjust volume to 50 mL Total n/a 50 mL Add in the order listed. Prepare in a 50 mL conical tube. Weigh LiDS and adjust the volume of DEPC-treated ultrapure H2O accordingly. Dissolve by incubating on a rotator at RT. Store at 4 °C. Shelf life is undetermined, > 6 months.
1% LiDS TE (50 mL)
Reagent Final concentration Amount or volume DEPC-treated ultrapure H2O n/a 44.4 mL 1 M Tris-HCl pH 8.0 10 mM 500 µL 0.5 M EDTA pH 8.0 1 mM 100 µL 10% LiDS m/v 1% m/v 5 mL Total n/a 50 mL Add in the order listed. Prepare in a 50 mL conical tube and store at RT. Shelf life is undetermined, > 1 year.
2% LiDS TE (2 mL)
Reagent Final concentration Amount or volume DEPC-treated ultrapure H2O n/a 1,552 µL 1 M Tris-HCl pH 8.0 20 mM 40 µL 0.5 M EDTA pH 8.0 2 mM 8 µL 10% LiDS m/v 2% m/v 400 µL Total n/a 2 mL Add in the order listed. Prepare in a 2 mL microcentrifuge tube and store at RT. Shelf life is undetermined, > 1 year.
10 M LiCl (50 mL)
Reagent Final concentration Amount or volume LiCl 10 M 21.20 g DEPC-treated ultrapure H2O n/a 39.76 mL Total n/a 50 mL Add in the order listed. Prepare in a 50 mL conical tube. Add LiCl quickly and close the cap. Adjust the volume of DEPC-treated ultrapure H2O accordingly to limit moisture absorption. Add water to LiCl salt while on ice. Close tube and incubate on ice with occasional inversion. Finish dissolving LiCl by incubating on a rotator at RT. Avoid exposing the tube seal to LiCl salt to prevent leakage. Clarify by centrifugation at 3,000–3,200× g for 30 min at RT, transfer 90% v/v of the clarified portion to a new 50 mL conical tube, and store at RT. Shelf life is undetermined, > 1 year.
Precipitation solution (40 mL)
Reagent Final concentration Amount or volume DEPC-treated ultrapure H2O n/a 5 mL 10 M LiCl 3.75 M 15 mL 100% isopropanol v/v 50% v/v 20 mL Total n/a 40 mL Add reagent components to DEPC-treated ultrapure H2O. Prepare in a 50 mL conical tube and store at RT. Shelf life is undetermined, > 1 year. A small decrease in volume to 36–38 mL after mixing components is expected.
95% MeOH (1 L)
Reagent Final concentration Amount or volume 100% MeOH v/v 95% v/v 950 mL DEPC-treated ultrapure H2O n/a 50 mL Total n/a 1 L Add in the order listed. Store in a glass airtight bottle and/or aliquot into 50 mL conical tubes. Store in flammable storage cabinet when not in use. Shelf life is undetermined, > 1 year. Alternatively, add 210.5 mL of DEPC-treated ultrapure H2O to an unopened 4 L bottle of MeOH.
Laboratory supplies
15 cm culture plates (e.g., Corning, catalog number: 430599)
Cell lifter (e.g., BIOLOGIX, catalog number: 70-2180)
30 mL syringe (e.g., VWR, catalog number: 76124-668)
18–22 G hypodermic needles (e.g., BD, catalog number: 305187)
Sterile syringe filter w/ 0.2 µm cellulose acetate membrane (e.g., VWR, catalog number: 28145-477)
1.5 and 2.0 mL microcentrifuge tubes (1.5 mL tubes must have round bottoms) (e.g., VWR, catalog numbers: 490004-444, 525-1136)
0.2 mL thermocycler tubes (e.g., VWR, catalog number: 20170-012)
15 and 50 mL conical tubes (e.g., Corning, catalog numbers: 430790, 430828)
0.5 and 1 L airtight glass bottles (e.g., VWR, catalog numbers: 89000-238, 89000-240)
Whatman paper (e.g., Sigma-Aldrich, catalog number: WHA1001150)
10, 200, and 1 mL pipette tips (e.g., Genesee Scientific, catalog numbers: 24-121RL, 24-150RS, 23-165RL)
10, 100, 200, and 1,000 µL pipettes (e.g., Gilson or Eppendorf)
10 and 25 mL serological pipettes (e.g., Genesee Scientific, catalog numbers: 12-104, 12-106)
Pipette controller (e.g., Thermo Fisher Scientific, catalog number: 14-387-165)
Kimwipes (e.g., VWR, catalog number: 82003-820)
96-well microplate (e.g., VWR, catalog number: 82050-678)
Thermal adhesive sealing film (e.g., Thermo Fisher Scientific, catalog number: 08-408-240)
Nitrile gloves (e.g., Microflex, catalog number: 92-134)
Repeater pipette (e.g., Eppendorf, catalog number: 022260201)
5 mL Combitips (e.g., Sigma-Aldrich, catalog number: EP0030089456)
Equipment
UV crosslinker (e.g., Stratagene, model: Stratalinker UV 2400 Crosslinker)
Analytical balance (e.g., Mettler Toledo, model: ME54E)
Sample tube rotator (e.g., Fisher Scientific Hematology Chemistry Mixer, model: 346)
Tube rack (e.g., VWR, catalog number: 82010-750)
Refrigerated microcentrifuge (e.g., Eppendorf, model: 5417R)
Centrifuge with 15 mL and 50 mL conical tube holders (e.g., Beckman, model: CS-6R)
Thermocycler (e.g., Bio-Rad, model: T100 PCR Thermocycler)
Hot plate stirrer (e.g., Accuplate, model: D0310)
UV spectrophotometer (e.g., NanoDrop, model: ND-100)
Microplate reader (e.g., VersaMax, model: M5e)
Dry bath/heating block (e.g., Thermolyne, model: 17600)
Mini centrifuge (e.g., ISC BioExpress, model: C1301P-ISC)
37 °C incubator (e.g., Baxter Scientific Products, model: J1450-3)
4 °C refrigerator (e.g., Kenmore, model: 253.6880201E)
-20 °C freezer (e.g., Kelvinator, model: KFU21M7LW3)
-80 °C freezer (e.g., VWR, model: 5706)
Software and datasets
Microsoft Excel for Mac version 16.66.1
SoftMax Pro version 7.2
NanoDrop 1000 version 3.8.1
Procedure
文章信息
稿件历史记录
提交日期: Mar 30, 2024
接收日期: Jun 19, 2024
在线发布日期: Jul 5, 2024
出版日期: Jul 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Kristofich, J. and Nicchitta, C. V. (2024). Rapid and Efficient Isolation of Total RNA-Bound Proteomes by Liquid Emulsion–Assisted Purification of RNA-Bound Protein (LEAP-RBP). Bio-protocol 14(14): e5236. DOI: 10.21769/BioProtoc.5236.
分类
生物化学 > RNA > RNA-蛋白质相互作用
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









