- EN - English
- CN - 中文
Cardiac-Specific Gene Editing via an AAV9-Tnnt2-SaCas9-miR122TS Vector
基于AAV9-Tnnt2-SaCas9-miR122TS载体的心脏特异性基因编辑
(*contributed equally to this work) 发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5235 浏览次数: 2509
评审: Xiaokang WuPooja MukherjeeAnonymous reviewer(s)
Abstract
The adeno-associated virus serotype 9 (AAV9)-delivered gene expression driven by the cardiac troponin T (Tnnt2) promoter is broadly considered to be cardiac-specific. However, in cases where low AAV expression is sufficient to trigger a profound biological effect in CRISPR/Cas9 gene editing, the ectopic AAV9-Tnnt2 expression and gene editing in the liver becomes non-negligible. MicroRNA122 is a microRNA that is specifically expressed in the liver. The incorporation of the microRNA122 target sequence (miR122TS) into the 3' untranslated region (UTR) of the AAV transgene could reduce ectopic gene expression in the liver. Here, we provide a protocol for sgRNA design, plasmid construction, AAV packaging, and in vivo validation of a new AAV9-Tnnt2-SaCas9-miR122TS vector using publicly available materials and tools. The application of this new vector enables cardiac-specific gene editing while circumventing leakages in the liver.
Key features
• This protocol describes a detailed procedure to construct and validate AAV-based cardiac-specific gene editing in mice.
• MicroRNA-122 target sequences (miR122TS) in combination with a Tnnt2 promoter are used to enhance the cardiac specificity in genome editing.
• Amplicon sequencing analysis is applied to precisely and sensitively quantify the genome editing efficiency and tissue specificity in mice.
Keywords: Adeno-associated virus (腺相关病毒)Graphical overview
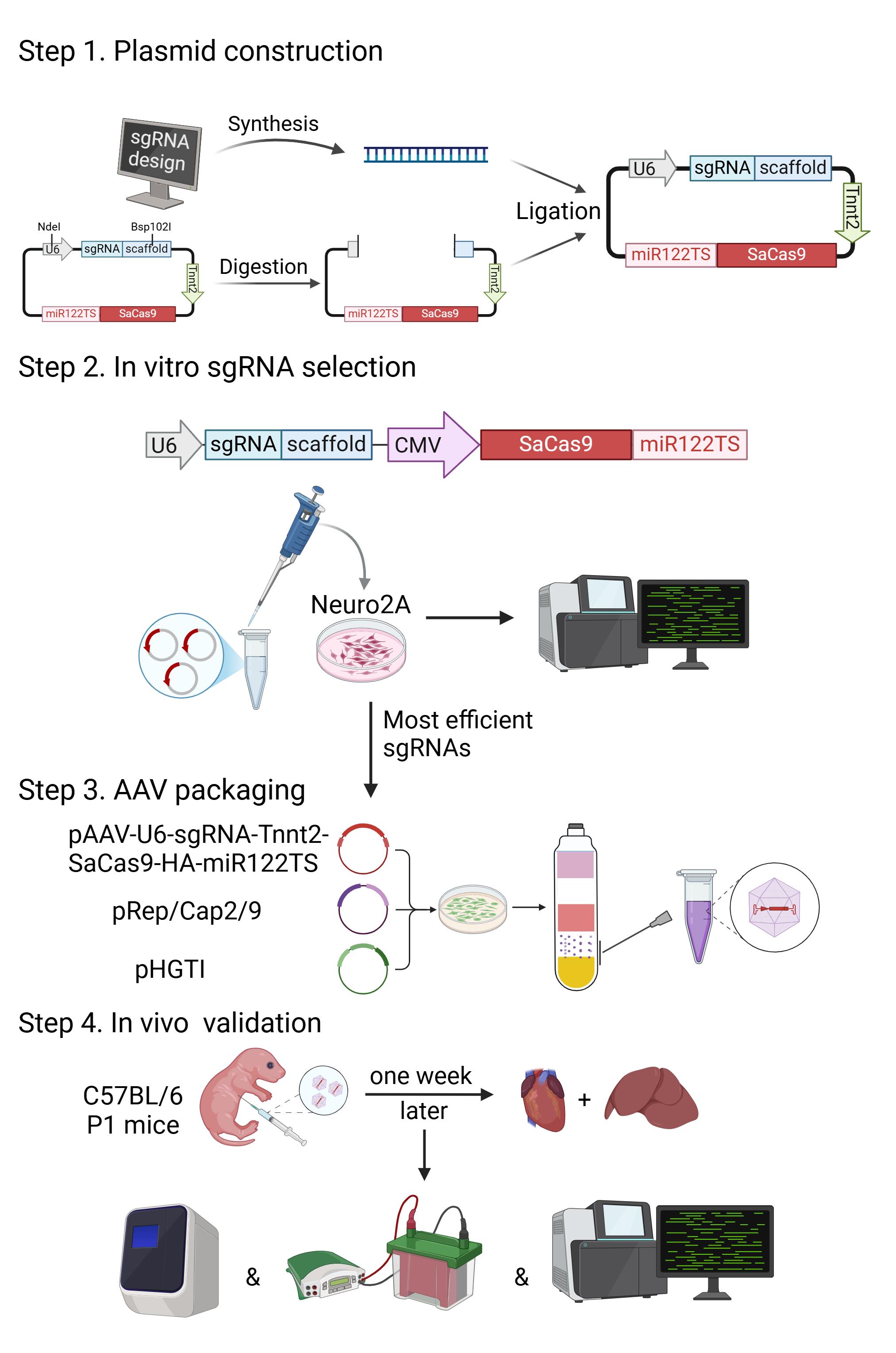
Construction and validation of AAV9-Tnnt2-SaCas9-miR122TS-based cardiac-specific gene editing. Step 1: In silico sgRNA design and plasmid construction. Step 2: sgRNA selection via gene editing in Neuro2A cells. Step 3: AAV packaging. Step 4: Validation of adeno-associated virus (AAV) expression, gene editing, and gene expression in murine tissues. P1, postnatal day 1. Graphics were generated with biorender.com.
Background
Adeno-associated virus (AAV) is a major gene delivery vector in basic and translational cardiology [1]. When a cardiac-specific promoter, such as the Tnnt2 promoter, is incorporated to drive gene expression via a cardiac tropic AAV capsid like AAV9, this technique is broadly believed to be cardiac-specific [2]. However, with increasingly favored AAV applications that could work under low expression levels, such as the Cre-LoxP and CRISPR/Cas9 genetic manipulation, the leaky expression of AAV9-Tnnt2 vector in the liver becomes non-negligible [3]. This caveat could potentially lead to data misinterpretation due to assumed cardiac specificity by AAV9-Tnnt2 vectors.
MicroRNAs are small non-coding RNAs that regulate gene expression at the post-transcriptional level. One major mechanism underlying the function of microRNA involves recognition and binding to complementary target sequences in mRNA [4]. This action leads to targeted mRNA cleavage followed by degradation and thereby gene silencing. Many microRNAs are expressed in a tissue-specific or cell type–specific manner and regulate key pathophysiological functions, which have been utilized to modulate exogenous transgene expression in a gene delivery system [5].
MicroRNA-122 is the most abundant microRNA that is specifically expressed in the liver [6]. Incorporation of microRNA-122 target sequence (miR122TS) in mRNA was known to exhibit a robust liver de-targeting effect on AAV-based gene transfer [7,8]. However, with the wide application of cardiac-specific promoters, the necessity of adding miR122TS into cardiac-targeted AAV vectors was underestimated. Recently, we showed that miR122TS was both necessary and sufficient to avoid hepatic leaky expression by the AAV9-Tnnt2 vector, which was of particular importance in Cre-LoxP and CRISPR/Cas9 applications that could work at a very low expression level [3]. Here, we describe how to design, construct, and validate an AAV9-Tnnt2-SaCas9-miR122TS vector to achieve cardiac-specific genome editing in mice with publicly available materials. Similar strategies could be applied to other efforts in cardiac-specific AAV gene transfer.
Materials and reagents
Animals
Studies described here were in conformity with the protocol authorized by the IACUC of Peking University with the approval number DLASBD0203. C57BL/6 mice were obtained from the Department of Laboratory Animal Science of Peking University Health Science Center.
For AAV injection, animals were anesthetized by isoflurane (RWD Life Science, catalog number: R510-22). Then, a 29G-needle insulin syringe (BD Ultra-FineTM, catalog number: 320312) was used to inject AAV via either subcutaneous or venous routes.
Plasmids
1. pAAV-U6-sgRNA-Tnnt2-SaCas9-HA-miR122TS (AAV vector plasmid) (Addgene, #209783)
2. pHGTI (AAV packaging helper plasmid) (Addgene, #112867)
3. pRepCap2/9 (AAV replication and capsid plasmid) (Addgene, #112865)
4. pAAV-CMV-GFP-LA (Addgene, #206197)
Cell lines
1. HEK293T cell (Wuhan Pricella Biotechnology, CL-0005)
2. Neuro2A cell (Wuhan Pricella Biotechnology, CL-0168)
Reagents
For plasmid construction
1. LB broth (Sangon Biotech, catalog number: A507002)
2. Ampicillin (Sangon Biotech, catalog number: B541011)
3. TIANprep Mini Plasmid kit (Tiangen, catalog number: DP103)
4. Stbl4 competent cells (Acmec, catalog number: AC10839-20×100 μL)
5. EndoFree Maxi Plasmid kit (Tiangen, catalog number: GDP117)
6. Genomic DNA extraction kit (Tiangen, catalog number: DP304)
7. E.Z.N.ATM Endo-Free Plasmid Giga kit (Omega, catalog number: D6234-01)
8. NdeI (Transgene, catalog number: JN201-01)
9. Bsp120I (Thermo, catalog number: ER0131)
10. Seamless Cloning kit (Sangon, catalog number: B632219)
11. 2× Taq DNA polymerase (Tiangen, catalog number: ET101)
12. 10× rCutsmart buffer (New England Biolabs, catalog number: B6004V)
For cell culture
1. DMEM (Gibco, catalog number: C11995500BT)
2. Fetal bovine serum (FBS) (Vistech, catalog number: SE100-B)
3. Penicillin-streptomycin (P/S) (TransGen, catalog number: FG101-01)
4. Phosphate-buffered saline (PBS) (FreeMoreBio, catalog number: FM58031)
5. 0.25% Trypsin-EDTA (Macgene, catalog number: CC017)
6. Opti-MEM (Cellworld, catalog number: c2656-862)
7. Polyetherimide (PEI), 1 mg/mL (Yeasen, catalog number: 40816ES03)
8. Lipo8000 transfection reagent (Beyotime, catalog number: C0533)
For AAV purification and analysis
1. Optiseal ultracentrifuge tube (Beckman, catalog number: 361625)
2. Amicon ultra centrifugal filter, 100 kDa MWCO (Millipore, catalog number: UFC9100)
3. Luer lock syringe (DengMiBio, catalog number: 302149)
4. Polyethylene glycol 8000 (PEG 8000) (Yeasen, catalog number: 60304ES76)
5. NaCl (HarveyBio, catalog number: SR5029)
6. 1 M Tris-HCl pH 8.0 (Yuanye, catalog number: R21105-500mL)
7. MgCl2 (HarveyBio, catalog number: SR4155)
8. KCl (HarveyBio, catalog number: SR1743)
9. 10× PBS (HarveyBio, catalog number: CT0035042)
10. Optiprep (Gibco, catalog number: 31985062)
11. Phenol red (Yuanye, catalog number: R22048)
12. Benzonase (Yeasen, catalog number: 20156ES60)
13. PluronicTM F-68 (Gibco, catalog number: 24040032)
14. DNase I (NEB, catalog number: M0303S)
15. Proteinase K (Beyotime, catalog number: ST532)
16. 10× reaction buffer (NEB, catalog number: B0303S)
For amplicon sequencing
1. TIANamp Genomic DNA kit (Tiangen, catalog number: GDP304-03)
2. TansNGC Dual Index Primers kit for Illumina (TransGen Biotech, catalog number: KI231)
3. Agarose (GenStar, catalog number: VA10252)
4. 50× TAE DNA electrophoresis buffer (GenStar, catalog number: E102-50)
For RT-qPCR
1. TransZol Up Plus RNA kit (TransGen Biotech, catalog number: ER501-01)
2. Taq Pro Universal SYBR qPCR master mix (Vazyme, catalog number: Q712-02)
3. HiScript III All-in-one RT SuperMix perfect for qPCR (Vazyme, catalog number: R333-01)
For western blot
1. SurePAGETM, Bis-Tris, 4%–12% (GenScript, catalog number: M00654)
2. Tris-MOPS-SDS (Shanghai yuanye Bio-Technology, catalog number: R23170)
3. 4× SDS-PAGE loading buffer (Solarbio, catalog number: P1016)
4. InStabTM protease cocktail (Yeasen, catalog number: 20124ES03)
5. Tween 20 (Sigma-Aldrich, catalog number: STS0200)
6. 0.45 μm PVDF membrane (Merck, catalog number: IPVH00010)
7. CaMKII delta antibody (GeneTex, catalog number: GTX111401)
8. HA-Tag antibody (Cell Signaling Technology, catalog number: 3724)
9. GAPDH antibody (TransGen Biotech, catalog number: HC301-01)
10. HRP-conjugated goat anti-rabbit IgG (TransGen Biotech, catalog number: HC101-01)
11. HRP-conjugated goat anti-mouse IgG (TransGen Biotech, catalog number: HC201-01)
12. Super ECL detection reagent (Yeasen, catalog number: 36208ES76)
13. Methyl alcohol (TGREAG, catalog number: 105099)
Solutions
1. Escherichia coli culture medium (see Recipes)
2. Complete mammalian cell culture medium (see Recipes)
3. Serum-free cell culture medium (see Recipes)
4. AAV precipitation solution (see Recipes)
5. Cell lysis buffer (see Recipes)
6. Iodixanol gradient (see Recipes)
7. AAV washing buffer (see Recipes)
8. RIPA buffer (see Recipes)
9. 5× transfer buffer (see Recipes)
10. 10×TBS (see Recipes)
Recipes
1. Escherichia coli culture medium (1 L)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| LB broth | 25 g/L | 25 g |
| Ampicillin | 100 mg/mL | 1 mL* |
| ddH2O | n/a | up to 1 L |
*Note: LB broth dissolved in water should be sterilized via a pressure steam sterilizer. Ampicillin should be added after the solution has cooled down to 50–60 °C.
2. Complete mammalian cell culture medium (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM | n/a | 445 mL |
| FBS | 10% (v/v) | 50 mL |
| P/S | 1% (v/v) | 5 mL |
3. Serum-free cell culture medium (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM | n/a | 495 mL |
| P/S | 1% (v/v) | 5 mL |
4. AAV precipitation solution (1 L)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PEG 8000 | 40% (w/v) | 400 g* |
| NaCl | 2.5 M | 146.25 g |
| ddH2O | n/a | up to 1 L |
*Note: PEG 8000 makes the large quantity of NaCl difficult to dissolve, thus put the mixture in a 37 °C water bath overnight or heat the mixture above 50 °C for 2 h.
5. Cell lysis buffer (50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl pH 8.0 | 20 mM | 1 mL |
| 5 M NaCl | 150 mM | 1.5 mL |
| 1 M MgCl2 | 1 mM | 50 μL |
| ddH2O | n/a | up to 50 mL |
Note: Filter the solution and store it at 4 °C.
6. Iodixanol gradient (50 mL)
| % of iodixanol | 10× PBS (mL) | 1 M MgCl2 (mL) | 5 M NaCl (mL) | 1 M KCl (mL) | Optiprep (mL) | Phenol red (mL) | ddH2O (mL) |
|---|---|---|---|---|---|---|---|
| 17 | 5 | 0.05 | 10 | 0.125 | 12.5 | 0 | 22.3 |
| 25 | 5 | 0.05 | 0 | 0.125 | 20 | 0.5 | 24.325 |
| 40 | 5 | 0.05 | 0 | 0.125 | 33.3 | 0 | 11.525 |
| 60 | 0 | 0.05 | 0 | 0.125 | 50 | 0.5 | 0 |
Note: Filter and store at 4 °C.
7. AAV washing buffer (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS | n/a | 500 mL |
| F-68 | 0.001% | 50 μL |
Note: Filter and store at 4 °C.
8. RIPA buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl pH 8.0 | 25 mM | 1.25 mL |
| 5 M NaCl | 150 mM | 1.5 mL |
| 10% SDS | 0.1% | 500 μL |
| 10% sodium deoxycholate | 0.5% | 2.5 mL |
| 10% Triton X-100 | 1% | 5 mL |
| ddH2O | n/a | Up to 50 mL |
Note: Add Protease Cocktail at 1:100 before use. Sterilize with 0.22 μm filter and store at -20 °C.
9. 5× transfer buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris | 0.125 M | 15.14 g |
| Glycine | 1 M | 75.07 g |
| ddH2O | n/a | Up to 1 L |
Note: Dilute to 1× transfer buffer with ddH2O. Add methyl alcohol at 1:4 before use.
10. 10×TBS
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 1.37 M | 80 g |
| KCl | 27 mM | 2 g |
| Tris | 25 mM | 30 g |
| ddH2O | n/a | Up to 1 L |
Note: Dilute to 1× TBS with dd H2O. Add Tween 20 at 1:1,000 before use.
Equipment
1. Water bath (Bluepard, model: HWS-12)
2. Dry thermostat (Kylin-Bell, model: GL-1900)
3. Constant temperature incubator (Bluepard, model: DHP-9032B)
4. Shaking incubator (ZhiCheng, model: ZWYR-211C)
5. Vortex mixer (Qlinbeier, model: VORTEX-6)
6. Heating plate with magnetic stirrer (Weibosci, model: 3483949)
7. Benchtop centrifuge (SCILOGEX, model: SCI-24)
8. High-speed centrifuge (Thermo Fisher, model: Sorvall RC 6 plus, 46910)
9. Ultracentrifuge (Beckman, model: Optima XPN Series, A99846)
10. CO2 incubator (ESCO, model: CLM-170B-8-NF)
11. All-in-one fluorescence microscope (Keyence, model: BZ-X800)
12. Autoclave sterilizer (ZEALWAY, model: GR85SA)
13. Vertical electrophoresis tank (Junyi Electrophoresis, model: JY-SCZ2+)
14. Horizontal electrophoresis tank (Junyi Electrophoresis, model: JY-SPCT)
15. AriaMx Real-Time PCR system (Agilent Technologies, model: AriaMx)
16. PCR thermal cycler (LongGene, model: T30D)
Software and datasets
1. CRISPick (https://portals.broadinstitute.org/gppx/crispick/public) (for sgRNA design)
2. Benchling (https://www.benchling.com/) (for vector cloning and primer design)
3. CRISPresso2 (https://github.com/pinellolab/CRISPResso2) (for amplicon sequencing analysis)
Procedure
文章信息
稿件历史记录
提交日期: Dec 1, 2024
接收日期: Feb 4, 2025
在线发布日期: Feb 25, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Yang, L., Guo, C., Sun, Y. and Guo, Y. (2025). Cardiac-Specific Gene Editing via an AAV9-Tnnt2-SaCas9-miR122TS Vector. Bio-protocol 15(5): e5235. DOI: 10.21769/BioProtoc.5235.
分类
发育生物学 > 基因组编辑 > 靶向整合
生物科学 > 生物技术 > CRISPR/Cas9
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












