- EN - English
- CN - 中文
Procedure for Reliable and Long-Lasting Ex Vivo Recordings of Sciatic Nerve Activity in Mice
小鼠坐骨神经活动的可靠且持久的离体记录方法
发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5233 浏览次数: 2526
评审: Mayank GautamAnonymous reviewer(s)
Abstract
Changes in neuronal conduction are common in disease states affecting peripheral nerves. These alterations can significantly impact nerve function and lead to sensorimotor disabilities. In vivo electromyography recording is a well-established electrophysiological method that has been used for decades to assess sensory and motor functions in the nervous system. Nerve studies are challenging to conduct in vivo in rodents, and the involvement of muscle activity makes it difficult to isolate and assess nerve function independently. This protocol provides a comprehensive guide for accurate ex vivo sciatic nerve dissection and handling from mice. It includes the creation of a three-compartment chamber and the establishment of electrophysiological protocols, which enable differential recordings and the analysis of compound action potentials from various nerve fibers. This setup allows researchers to study the specific effects of drugs and pathologies on nerves from a mechanistic perspective. The setup is a stand-alone apparatus that does not require the use of suction electrodes and the maintenance of negative pressure, which can affect the signal-to-noise ratio and recording stability.
Key features
• Sciatic nerve electrophysiology recordings from mice.
• Allows for testing of disease model effects.
• Ex vivo setup enables accurate pharmacological tests.
• User-friendly software acquisition and analysis of compound action potential response.
Keywords: Sciatic nerve (坐骨神经)Graphical overview
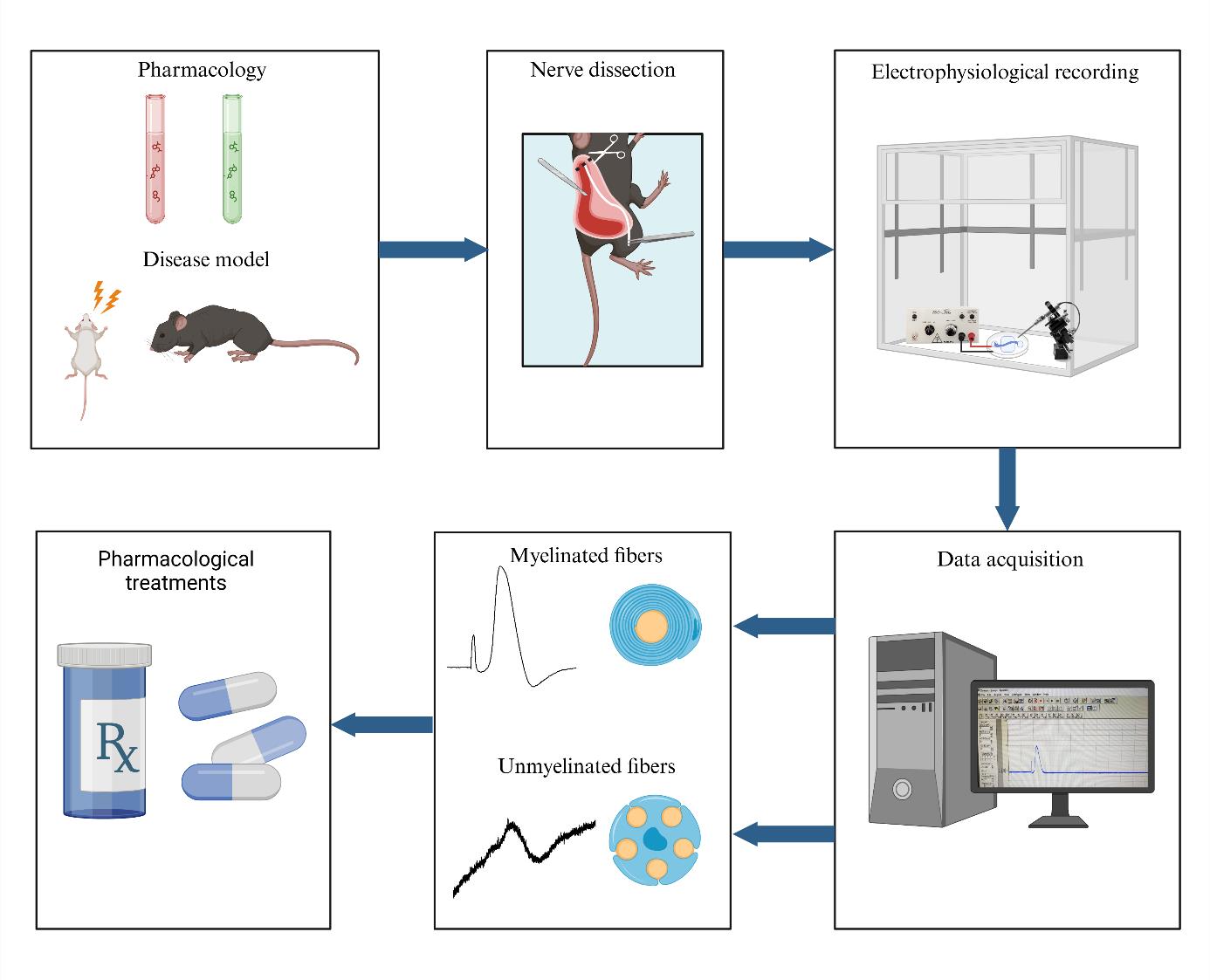
Background
Peripheral nerve function is compromised in a range of disorders, including diabetic neuropathy and autoimmune conditions like Guillain-Barré syndrome and chronic inflammatory demyelinating polyneuropathy, as well as hereditary diseases such as Charcot-Marie-Tooth disease [1–4]. Injuries, infections (e.g., Lyme disease, Mycobacterium leprae), vitamin deficiency, and intoxications (e.g., heavy metals) may also lead to nerve damage [3,5,6]. Depending on which nerves and nerve fibers are affected, the symptoms can include pain, numbness, tingling, and weakness, and can alter sensory, motor, or autonomic functions in the body [7]. Nociceptors detect harmful stimuli like extreme temperature, pressure, or injury-related chemicals in the skin. The signal is then converted into electrical impulses sent to the brain, shaping the diverse qualities of pain [8,9].
In vivo electromyography is a long-established method for assessing sensory and motor functions in the peripheral nervous system [10,11]. Conducting nerve studies in live rodents poses several challenges. Muscle activity is often involved, making it difficult to isolate and assess nerve function independently—an important distinction when evaluating effects on nerves vs. muscles. Another challenge with in vivo electrophysiological setups is the impact of anesthetics and blood perfusion, which can interfere with direct pharmacological effects or influence nerve activity. Maintaining physiological temperature is also essential in these setups, as temperature strongly modulates nerve conduction. Finally, the use of a smaller device and low-volume chamber helps minimize noise within the system, improving control and accuracy.
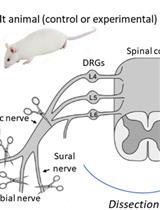
An ex vivo electrophysiology setup for the isolated sciatic nerve is a vital tool in neuroscience research, offering significant insights into nerve function and pathology. The use of suction electrodes, which gently capture nerve fibers to record action potentials is increasingly used to study ex vivo peripheral nerve function [12,13]. However, suction electrodes can be technically difficult to prepare and use. Also, potential amplitude and recording stability strongly depend on both the quality of the suction maintenance and the nerve/electrode resistance [14]. An alternative is the use of a Vaseline-sealed three-chamber-compartment recording technique (Figure 1). This setup enables precise recording of electrical activity from the isolated mouse sciatic nerve, facilitating the study of various disease models and the effects of pharmacological manipulations. Numerous animal models are available to investigate myelin biology, conduction, and the pathological mechanisms underlying conduction defects in peripheral neuropathies. While electromyographic recording offers limited insights into both myelinated and unmyelinated fiber populations, ex vivo recordings of nerve trunks provide a valuable opportunity to examine specific alterations in axonal populations and conduct selective pharmacological studies. This electrophysiological setup evaluates the conduction properties of the sciatic nerve by stimulating the distal end, replicating pain-related signals, and enabling functional analysis of nerve fibers (Figure 1). Key findings include: (1) Conduction velocity: differentiates large fibers (Aβ, Aδ) from small fibers (C), essential for studying nociceptive and mechanosensitive pathways. (2) Amplitude: reflects nerve fiber activity and recruitment potential, which can be altered under conditions such as pain or inflammation. (3) Refractory period: reveals nerve recovery and firing capacity, critical for understanding pain-related repetitive firing. (4) Excitability changes: hyper- and hypo-excitability, which are linked to allodynia and hypoesthesia. By maintaining the viability of the isolated nerve for extended periods, researchers can conduct long-term experiments, enabling detailed investigations into nerve conduction and the impact of therapeutic interventions. This capability is essential for advancing our understanding of neurological diseases and developing new treatments.
Materials and reagents
Biological materials
1. C57BL/J6 or ICR 3–6-month-old mice (Harlan Laboratories)
Reagents
1. Potassium chloride (KCl) (Sigma-Aldrich, catalog number: 1.04936)
2. Magnesium sulfate (MgSO4) (Bio Lab, catalog number: 13190391)
3. Sodium monophosphate (NaH2PO4) (Bio Lab, catalog number: 1004921-5)
4. Sodium chloride (NaCl) (Bio Lab, catalog number: 1903059)
5. Sodium bicarbonate (NaHCO3) (Frutarom, catalog number: 5553350)
6. Glucose (C6H12O6) (Sigma-Aldrich, catalog number: 1.0837)
7. Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: 1.02382)
8. Pentobarbital (CTS Chemical Industries LTD, catalog number: 2241702)
Solutions
1. Stock solutions: 300 mM KCl stock solution, 200 mM MgSO4 stock solution, 125 mM NaH2PO4 stock solution, 200 M CaCl2 stock solution, 1,260 mM NaCl stock solution, and 260 mM NaHCO3 stock solution (see Recipes)
2. Artificial cerebrospinal fluid (aCSF) (see Recipes)
Recipes
1. Stock solutions
| Reagent | Final concentration | Quantity | Volume |
| KCl | 300 mM | 1.12 g | 50 mL |
| MgSO4·7H2O | 200 mM | 2.46 g | 50 mL |
| NaH2PO4·H2O | 125 mM | 0.86 g | 50 mL |
| CaCl2·2H2O | 200 mM | 1.47 g | 50 mL |
| NaCl | 1,260 mM | 36.82 g | 500 mL |
| NaHCO3 | 260 mM | 10.92 g | 500 mL |
Stocks should be prepared in double-distilled water (DDW) and then filtered. Filtered stocks should be stored at 4 °C for three months and checked periodically for contamination.
2. Artificial cerebrospinal fluid (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 300 mM KCl stock solution | 3 mM | 5 mL |
| 200 mM MgSO4 stock solution | 2 mM | 5 mL |
| 125 mM NaH2PO4 stock solution | 1.25 mM | 5 mL |
| 1,260 mM NaCl stock solution | 126 mM | 50 mL |
| 260 mM NaHCO3 stock solution | 26 mM | 50 mL |
| Glucose | 10 mM | 0.9008 g |
| 200 mM CaCl2 stock solution | 2 mM | 5 mL |
| DDW | n/a | see note* |
| Total | n/a | 500 mL |
On the day of the preparation:
Pour 350 mL of DDW into a beaker containing a stirring bar. Add the KCl, NaH2PO4, MgSO4, NaCl, and NaHCO3 from the prepared stocks. Measure the glucose and add it to the solution.
Note: Do not add CaCl2 at this point as it could precipitate. Continue stirring.
When all powders have dissolved, cover the beaker with parafilm. Oxygenate the solution. Place a clean tube connected to the 95% O2, 5% CO2 gas tank into the bottle. After 30 min of oxygenation, add CaCl2 from the stock, adjust the pH to 7.4 using NaOH, and add DDW to complete the volume to 500 mL. Stir and keep oxygenated.
Laboratory supplies
1. Spray bottle with 70% ethanol (Vitamed, catalog number: 7290008082990)
2. 100% ethanol (Sigma-Aldrich, catalog number: 459844)
3. Petri dish 60 mm × 15 mm (Sigma-Aldrich, catalog number: P5481)
4. Sylgard 184 (Dow Corning, catalog number: 3097366-0516, 3097358-1004)
5. Heavy-duty silicone grease (Molykote, Dupont, catalog number: Z273554)
6. Three-compartment chamber (created in-house; see Section A)
7. 95% O2 and 5% CO2 gas mixture
Equipment
Dissection and nerve isolation
1. Surgical scissors (Fine Science Tools, catalog number: 14200-21)
2. Extra fine Bonn scissors (Fine Science Tools, catalog number: 14084-08)
3. Tissue forceps (Fine Science Tools, catalog number: 11021-12)
4. N° 3 scalpel handle (Sigma-Aldrich, catalog number: S2896)
5. Size 11 carbon steel surgical blade (Swann Morton, Sheffield, England, catalog number: 0203)
6. Vannas scissors (Surgitrac, catalog number: SC81)
7. Straight tying forceps (Surgitrac, catalog number: SC19)
8. Surgical marker (Stericlin, catalog number: 210110)
9. Jeweler’s forceps (Surgitrac, catalog number: SC60)
Electrophysiology
1. Digital stereomicroscope (Nikon, model: SMZ745T)
2. Light source, 24 V, 150 W (MXBAOHENG, model: XD-301)
3. Amplifier with headstage (NPI, model: EXT-02F – Extracellular Amplifier)
4. Digitizer (Molecular Devices, model: Digidata 1440A)
5. Current stimulator (AMPI, Isoflex)
6. Faraday cage (WPI, model: AT-3648-FCB)
7. Bare silver wire (diameter: 1 mm; length: 30 mm) for recording and stimulation electrodes (WPI, model: AGW4010)
8. 1 mm × 2.5 mm Ag/AgCl pellet with 70 mm embedded Ag wire (NPI, catalog number: ALA-P-BMP-1)
9. Banana plugs and cable (RS, catalog number: 261-6518)
10. BNC cables (RS, catalog number: 122-2144)
11. PC computer (HP, model: EliteDesk 800 G2 TWR)
12. Four 17.5 mm Eclipse magnets with M6 threaded hole (RS, catalog number: 298-0863)
13. Four M6 screws and bolts (RS, catalog number: 277-604)
14. Warming platform for Petri dish (NPI, model: TC-10/20 Temperature Controller)
15. Temperature controller (NPI, model: TC-10/20 Temperature Controller)
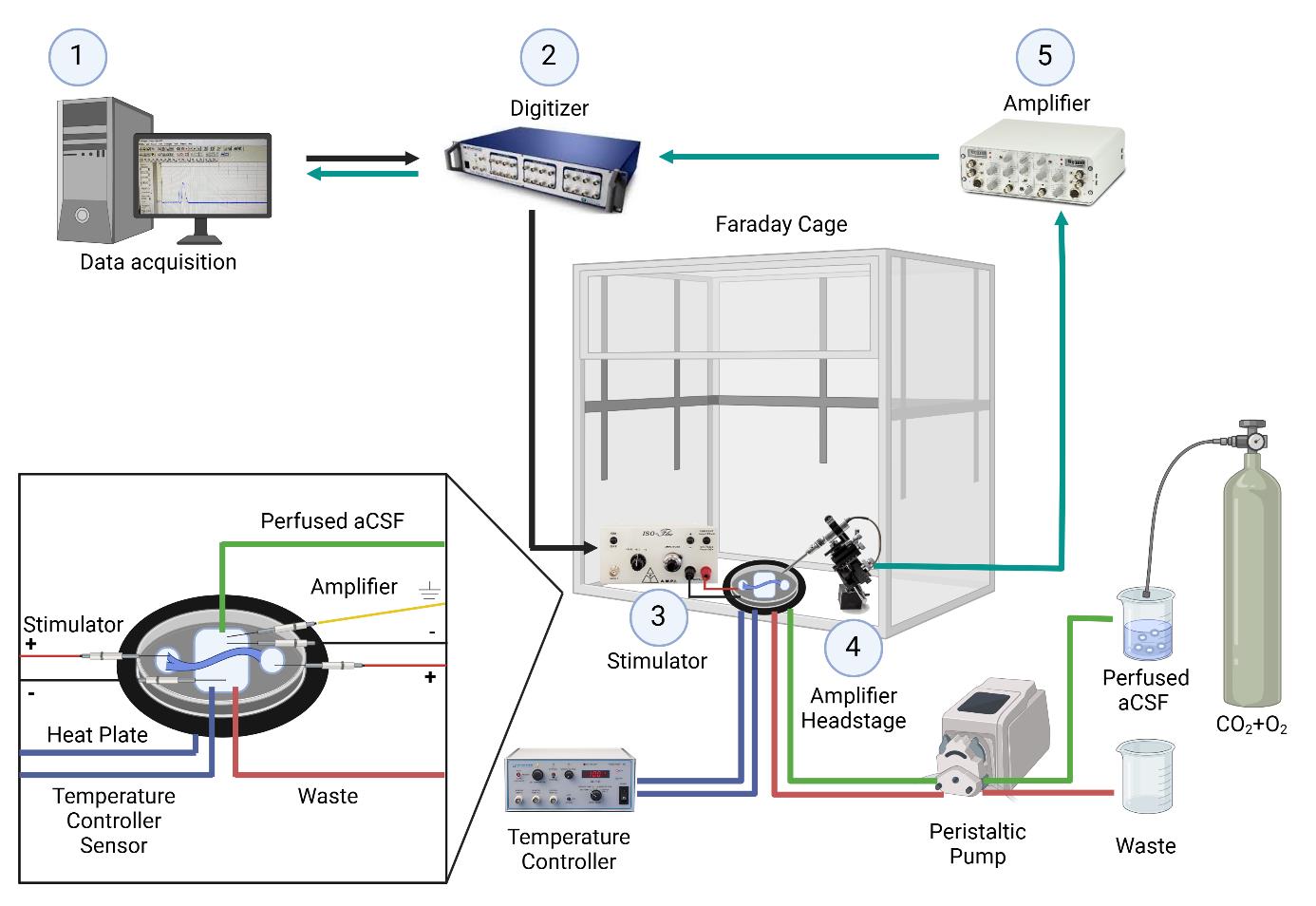
Figure 1. Electrophysiology setup for ex-vivo sciatic nerve recordings. The nerve is placed in a temperature-controlled chamber with continuously perfused artificial cerebrospinal fluid. 1. Data is acquired and analyzed using Clampex software. 2. A command signal is sent from the computer to the digitizer, which is then relayed from the digitizer to the stimulator. 3. Nerve stimulation occurs through a pair of electrodes connected to the stimulator and placed in the left well (positive electrode) and middle chamber (negative electrode). 4. The signal is acquired through the amplifier headstage connected to a pair of recording electrodes placed in the middle chamber (negative electrode) and the well on the right side (positive electrode). 5. The signal is then amplified. After amplification, the signal is sent to the digitizer and back to the computer. Created with BioRender.com.
Software and datasets
1. pClamp (version 10.7, July 2016, Molecular Devices)
2. Clampfit (version 11.3, September 2023, Molecular Devices)
Note: The software requires a license (Molecular Devices).
Procedure
文章信息
稿件历史记录
提交日期: Nov 16, 2024
接收日期: Feb 4, 2025
在线发布日期: Feb 25, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Berkowitz, S., Goldberg, Z., Dori, A., Maggio, N., Shavit-Stein, E. and Devaux, J. J. (2025). Procedure for Reliable and Long-Lasting Ex Vivo Recordings of Sciatic Nerve Activity in Mice. Bio-protocol 15(5): e5233. DOI: 10.21769/BioProtoc.5233.
分类
神经科学 > 神经解剖学和神经环路 > 坐骨神经
神经科学 > 周围神经系统 > 坐骨神经
生物物理学 > 电生理
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link