- EN - English
- CN - 中文
Protocol for Screening Host-Targeting Antivirals (HTAs) Using Human PBMCs and pDCs
基于人外周血单个核细胞(PBMCs)和浆细胞样树突细胞(pDCs)的宿主靶向抗病毒药物(HTA)筛选方案
发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5230 浏览次数: 3050
评审: Luis Alberto Sánchez VargasAndrew SylwesterPaurvi Shinde

相关实验方案
用于比较人冷冻保存 PBMC 与全血中 JAK/STAT 信号通路的双磷酸化 CyTOF 流程
Ilyssa E. Ramos [...] James M. Cherry
2025年11月20日 2286 阅读
Abstract
This protocol offers an ex vivo method for screening host-targeting antivirals (HTAs) using human peripheral blood mononuclear cells (PBMCs) or plasmacytoid dendritic cells (pDCs). Unlike virus-targeting antivirals (VTAs), HTAs provide advantages in overcoming drug resistance and offering broad-spectrum protection, especially against rapidly mutating or newly emerging viruses. By focusing on PBMCs or pDCs, known for their high production of humoral factors such as Type I interferons (IFNs), the protocol enables the screening of antivirals that modulate immune responses against viruses. Targeting host pathways, especially innate immunity, allows for species-independent antiviral activity, reducing the likelihood of viral escape mutations. Additionally, the protocol's versatility makes it a powerful tool for testing potential antivirals against various viral pathogens, including emerging viruses, positioning it as an essential resource in both pandemic preparedness and broad-spectrum antiviral research. This approach differentiates itself from existing protocols by focusing on host immune modulation through pDCs, offering a novel avenue for HTA discovery.
Key features
• Optimized protocol for screening HTAs against dengue virus (DENV), chikungunya virus (CHIKV), and Zika virus (ZIKV).
• This protocol is ideal for screening soluble or intravenous-formulated compounds for evaluating their efficacy in experimental settings.
• This protocol builds upon the method developed by Tsuji et al. [1] and extends its application to PBMCs and testing against DENV, CHIKV, and ZIKV.
Keywords: Host-targeting antivirals (HTA) (宿主靶向抗病毒药物(HTA))Graphical overview
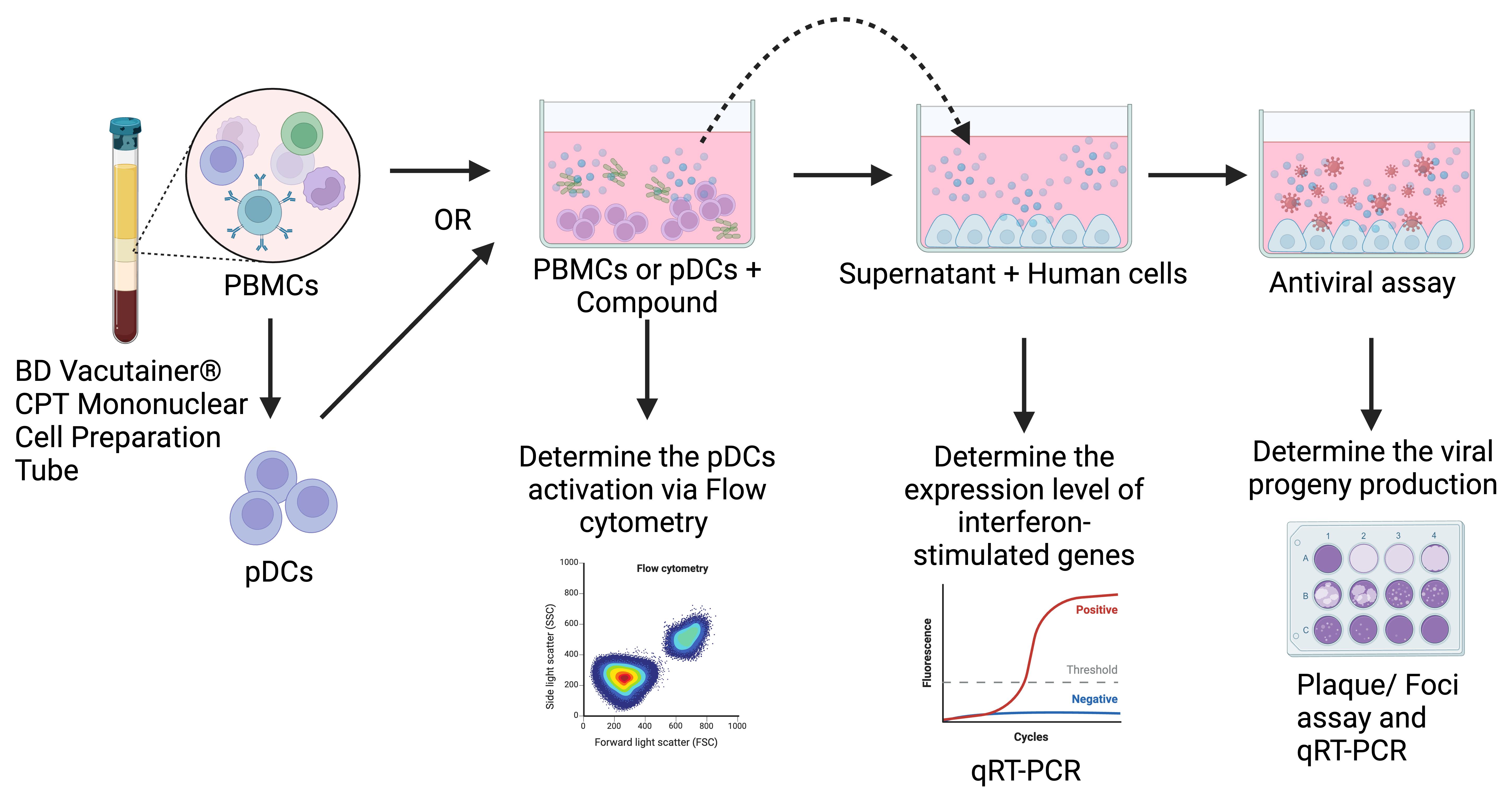
Background
The prevalence of arboviruses like dengue (DENV), chikungunya (CHIKV), and Zika (ZIKV) underscores the need for innovative antiviral strategies [2–4]. Traditionally, virus-targeting antivirals (VTAs) have focused on viral components [5–8], which often lack broad-spectrum efficacy and are prone to resistance from rapidly mutating viruses due to their specificity. Host-targeting antivirals (HTAs), which target host proteins or pathways essential for viral replication, offer a promising alternative [9], providing a higher genetic barrier to resistance and broad-spectrum activity. The FDA-approved Maraviroc medication for HIV-1 highlights the growing importance of HTAs in antiviral research [10]. This manuscript presents an ex vivo model to screen HTAs using immune cells like peripheral blood mononuclear cells (PBMCs) or plasmacytoid dendritic cells (pDCs). It builds upon the method developed by Tsuji et al. [1] where bone marrow–derived dendritic cells (bmDCs) were stimulated with HTAs. Here, we use PBMCs, directly removing the need for advanced technical expertise and experimental animals associated with isolating bmDCs. PBMCs, which include lymphocytes, monocytes, and dendritic cells, produce a wide range of immune factors, making them physiologically relevant for studying immune-modulating antivirals [11]. The protocol uses BD Vacutainer CPT mononuclear cell preparation tubes for quick and efficient PBMC isolation within 1 h while preserving cell integrity [12]. Since interferon (IFN) responses are critical to the first line of antiviral immunity, the protocol focuses on HTAs that activate isolated pDCs or pDCs within the PBMCs, which produce Type I interferons [13,14]. This method describes the isolation and culture of PBMCs or pDCs for HTA screening, involving the transfer of conditioned media from these immune cells to human liver cells (Huh-7) to assess the reduction in viral infectivity. It closely mimics physiological conditions, as HTAs in the bloodstream are likely to interact with immune cells like PBMCs or pDCs, producing humoral factors that travel to distant organs, and prime human cells for enhanced antiviral defense. Additionally, it introduces an immunophenotyping assay to assess the activation of pDCs and an IFN-stimulating genes (ISGs) expression study to evaluate the antiviral status of Huh-7 cells. These mechanistic assays are highly adaptable; while the current setup focuses on pDCs activation and ISGs induction, it can be modified to investigate other mechanisms by changing the primers used for gene expression analysis or selecting alternative immune cell surface markers to investigate other cells in the PBMCs such as monocytes and CD8+ T cells. This flexibility ensures that the protocol can be tailored to specific research needs, making it a versatile tool in the study of HTAs. While the protocol provides a strong platform for screening HTAs, it lacks the complexity of in vivo models, limiting the ability to study interactions between different immune cells in a systemic context [15]. The conditioned media approach offers an indirect assessment of antiviral activity, missing some cellular interactions and immune responses seen in real infections. However, further in vivo evaluations using C57BL/6, AG129, and BALB/c are required to confirm the efficacy of the antiviral against DENV, CHIKV, and ZIKV before proceeding to clinical studies. Moreover, since immune cells must directly engage with the HTAs, tested drugs ideally need to be injectable to reach immune cells in the bloodstream. This limits the screening of oral or topical HTAs. However, repurposing injectable drugs with established safety profiles could expedite clinical use [16]. Future work should aim to address these limitations by incorporating in vivo models to provide a more comprehensive understanding of selected HTAs, investigating other immune pathways, and considering more diverse viral models and HTA candidates.
Materials and reagents
Biological materials
1. Human peripheral blood mononuclear cells (PBMCs) either isolated from healthy individuals or purchased from IQ Biosciences (catalog number: IQB-PBMC103) or ImmunoSpot by CTL (for specific PBMC selection, please visit https://immunospot.com/epbmc).
2. Vero African green monkey kidney cells (ECACC strain, ATCC CCL-81)
3. Huh-7 human hepatocytes (JCRB Cell Bank, JCRB0403)
4. DENV-2 (NGC, ATCC VR-1584)
5. CHIKV (ECSA, FN295485)
6. ZIKV (P6740, obtained from the University of Texas Medical Branch)
Reagents
1. Phosphate-buffered saline (PBS) (CANVAX, catalog number: BR0003)
2. Cellbanker2 (AMSBIO, catalog number: 11914)
3. Roswell Park Memorial Institute (RPMI) 1640 [+] l-glutamine (Corning, catalog number: MT-10-040-CV)
4. Sodium pyruvate (1 mM) (Gibco, catalog number: 11360-070)
5. 0.25% 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (2.5 mM) (Gibco, catalog number: 15630-080)
6. Antibiotic-antimycotic (Gibco, catalog number: 15240-062)
7. Fetal bovine serum (FBS) (Gibco, catalog number: 10270-106)
8. Non-essential amino acid (NEAA) (Gibco, catalog number: 11140-050)
9. Dulbecco’s modified Eagle’s medium (DMEM) [+] 4.5 g/L glucose, l-glutamine, sodium pyruvate (Corning, catalog number: MT-10-013-CV)
10. Dulbecco’s modified Eagle’s medium (DMEM) [+] 1.5 g/L glucose, l-glutamine, sodium pyruvate (Corning, catalog number: MT-10-009-CV)
11. DMEM, powder, high glucose (Gibco, catalog number: 12100046)
12. Trypsin powder (Gibco, catalog number: 27250018)
13. Oligodeoxynucleotides (ODNs) containing unmethylated cytosine-guanine dinucleotides (CpG) (CpG ODN 2216) (InvivoGen, catalog number: tlrl-2216)
14. Pharmingen stain BSA buffer (staining buffer) (BD, catalog number: MAB554657)
15. CD304 HU NEUROPILIN-1 BB515 U21-1283 (BD, catalog number: PMG566036)
16. HU IL-3RALP (CD123) APC 6H6 (BD, catalog number: PMG567275)
17. HU CD86 PE IT2.2 (BD, catalog number: PMG555665)
18. ANTI-HLA-DR PE-CY7 L243 (G46-6) RUO/GMP (BD, catalog number: MAB335795)
19. 7-AAD viability dye, VIA-probe solution (BD, catalog number: MAB555816)
20. High viscosity carboxymethyl cellulose (CMC) (Sigma-Aldrich, catalog number: 9004-32-4)
21. Paraformaldehyde (MP Biomedicals, catalog number: 02150146-CF)
22. Triton X-100 (from any qualified supplier)
23. Skim milk (Chemiz, catalog number: 12947)
24. Anti-human HRP-conjugated secondary antibody IgG (H+L) (Invitrogen, catalog number: A18903)
25. TrueBlue peroxidase substrate (Seracare, catalog number: 5510-0030)
26. Crystal violet stain (from any qualified supplier)
27. RNeasy Kit (Qiagen, catalog number: QIAG-74106)
28. iScript cDNA Synthesis kit (Bio-Rad, catalog number: 1708891)
29. Luna universal qPCR master mix (New England BioLabs, catalog number: M3003L)
30. EasySep Human Plasmacytoid DC Isolation kit (STEMCELL Technologies, catalog number: 17977)
31. EasySep buffer (STEMCELL Technologies, catalog number: 20144)
Solutions
1. PBMC culture media (see Recipes)
2. Virus immobilizing media (see Recipes)
3. Cell culture growth media (see Recipes)
4. Cell culture maintenance media (see Recipes)
Recipes
1. PBMC culture media (50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Antibiotic-antimycotic (100×) | 1× | 500 μL |
| Sodium pyruvate (100 mM) | 1 mM | 500 μL |
| HEPES (1 M) | 2.5 mM | 125 μL |
| FBS (100%) | 10% | 5 mL |
| NEAA (100×) | 1× | 500 μL |
| RPMI 1640 medium (1×) | 1× | 43.375 mL (top up RPMI media to 50 mL) |
| Total | 50 mL |
2. Virus immobilizing media (500 mL)
Note: Dissolve 4.5 g of CMC in 250 mL of Milli-Q water. Autoclave the solution to sterilize and fully dissolve the CMC. Prepare 500 mL of 2× DMEM by dissolving one pack of DMEM powder in 500 mL of Milli-Q water, then filter to sterilize the media.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CMC (1.8%) | 0.9% | 250 mL |
| DMEM [+] 4.5 g/L glucose (2×) | 1× | 240 mL |
| FBS (100%) | 2% | 10 mL |
| Total | 500 mL |
3. Cell culture growth media (50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
DMEM [+] 4.5 g/L glucose, l-glutamine, sodium pyruvate (for Vero cells growth culture) (1×) Or DMEM [+] 1.5 g/L glucose, l-glutamine, sodium pyruvate (for Huh-7 cells growth culture) (1×) | 1× | 45 mL |
| FBS (100%) | 10% | 5 mL |
| Total | 50 mL |
4. Cell culture maintenance media (50 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM [+] 4.5 g/L glucose, l-glutamine, sodium pyruvate (for Vero cells maintenance culture) (1×) | 1× | 49 mL |
| FBS (100%) | 2% | 1 mL |
| Total | 50 mL |
Laboratory supplies
1. BD Vacutainer CPT mononuclear cell preparation tubes (8 mL per tube) (BD, catalog number: SS362761)
2. 20-gauge (0.9 mm) needle (from any qualified supplier)
3. 96-well plate (from any qualified supplier)
4. 24-well plate (from any qualified supplier)
5. 2 mL internal threaded polypropylene cryogenic vial, self-standing with round bottom (Corning, catalog number: 431386)
6. 15 and 50 mL centrifuge tubes (from any qualified supplier)
7. 1.5 mL tubes (Eppendorf, catalog number: 022363204)
8. Falcon 5 mL round-bottom polystyrene test tube, with cell strainer snap cap (Corning, catalog number: 352235)
Equipment
1. Inverted microscope (Olympus, model: CKX31)
2. TC20 automated cell counter (Bio-Rad, catalog number: 1450102)
3. Refrigerated centrifuge (Eppendorf, catalog number: 5804 000.017)
4. Class II biological safety cabinet (BSC) (ESCO, model: SC2-4E1)
5. Flow cytometer with blue and red laser (BD, model: FACSCanto II)
6. NanoDrop 2000/2000c spectrophotometer (Thermo Fisher Scientific, catalog number: ND2000CLAPTOP)
7. Applied BiosystemsTM SimpliAmpTM thermal cycler (Thermo Fisher Scientific, catalog number: A24811)
8. QuantStudio 5 real-time PCR system (Thermo Fisher Scientific, catalog number: A34322)
9. EasySep Violet Magnet (STEMCELL Technologies, catalog number: 18000)
Software and datasets
1. FACSDiva software (version 6.1.2, 23/09/2008)
2. Design & Analysis 1 (DA1) software (version 1.5.2)
3. EZR software program (version 1.63, 30/6/2024)
Procedure
文章信息
稿件历史记录
提交日期: Dec 4, 2024
接收日期: Feb 4, 2025
在线发布日期: Feb 25, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Low, Z. X., Kanauchi, O., AbuBakar, S., Tiong, V. and Hassandarvish, P. (2025). Protocol for Screening Host-Targeting Antivirals (HTAs) Using Human PBMCs and pDCs. Bio-protocol 15(5): e5230. DOI: 10.21769/BioProtoc.5230.
分类
免疫学 > 免疫细胞功能 > 细胞因子
免疫学 > 免疫机理 > 体外模型
药物发现 > 药物筛选
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link