- EN - English
- CN - 中文
Microbial Biofilm Detection and Differentiation by Dual Staining Using Maneval’s Stain
利用 Maneval 染色双重染色法检测和区分微生物生物膜
(§ Technical contact) 发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5228 浏览次数: 2570
评审: Emilia KrypotouRubikah VimonishAnonymous reviewer(s)
Abstract
Microbial biofilms are structured communities of microorganisms embedded in a self-produced extracellular matrix, adhering to surfaces. These biofilms enhance bacterial resistance to antibiotics, immune responses, and environmental stress. Current microscopy techniques, such as scanning electron microscopy (SEM), confocal laser scanning microscopy (CLSM), and fluorescence microscopy, are commonly used to visualize and differentiate biofilms. However, their high cost and complexity often render them impractical. In contrast, simpler methods like crystal violet and Congo red staining are limited in distinguishing bacterial cells from the biofilm matrix. This study introduces a cost-effective, dual-staining method using Maneval’s stain to visualize and differentiate microbial biofilms. It requires only basic equipment and minimal reagents, making it ideal for routine use in clinical diagnosis and microbial research.
Key features
• This dual-staining method differentiates bacterial cells, biofilm matrix, and capsules in a single stain.
• This method applies to both bacterial and fungal biofilm.
• This method requires no specialized training or equipment, with the entire process completed within 30–45 min.
• Stained slides can be stored for extended periods (months) without degradation.
Keywords: Bacterial biofilm (细菌生物膜)Graphical overview
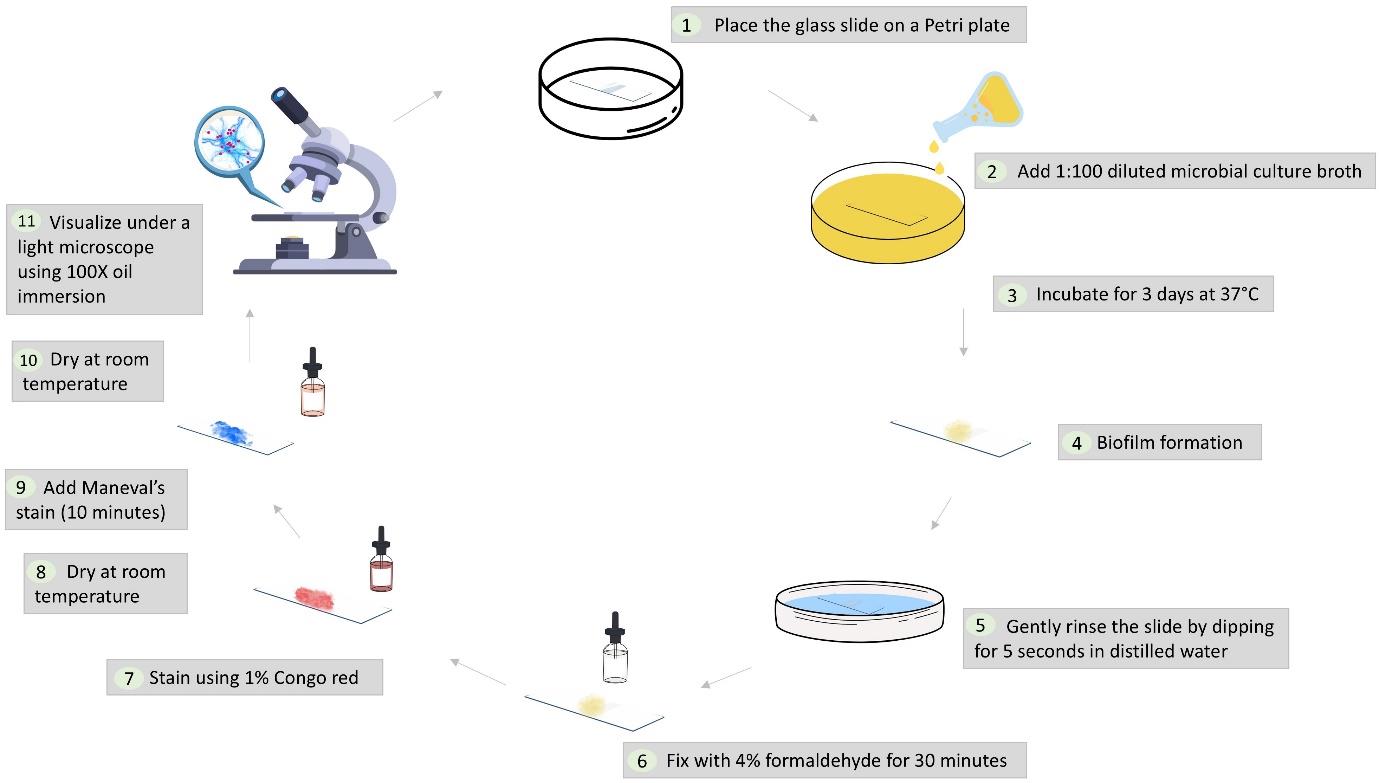
Workflow illustrating the dual-staining protocol for microbial biofilms using Maneval’s stain
Background
The discovery of biofilms dates back to the Dutch microscopist Antonie van Leeuwenhoek, who first observed them on tooth surfaces [1]. They were later studied by J.W. Costerton, who elucidated their role in persistent infections and antibiotic resistance [2]. These microbial biofilms are organized communities of microorganisms that adhere to surfaces and are encased in an extracellular matrix (ECM). The biofilm life cycle involves attachment, irreversible adhesion, maturation into microcolonies, and dispersion. The ECM, composed of polysaccharides, proteins, DNA, and lipids, acts as a protective shield, making biofilms highly resistant to antimicrobial treatments and immune responses [3].
Understanding biofilms is crucial for developing effective detection methods and treatments. Biofilm detection is crucial in clinical diagnosis, as biofilms contribute to antibiotic resistance and can lead to chronic conditions [4]. In the food industry, detecting biofilms on processing equipment is vital for preventing contamination and spoilage [5]. Monitoring biofilm growth in industrial water systems, cooling towers, and pipelines is essential to maintain operational efficiency [6]. Biofilm detection is important for studying microbial communities in natural environments, such as streams, soil, and marine ecosystems [7]. Moreover, pharmaceutical companies rely on biofilm detection methods to develop and assess the effectiveness of antibiofilm treatments [8].
Advanced microscopy techniques, such as scanning electron microscopy (SEM), confocal laser scanning microscopy (CLSM), and fluorescence microscopy, are commonly used to visualize and differentiate biofilms. However, these methods are not feasible in all laboratories because of their high cost and complexity [9]. In contrast, simpler methods like crystal violet and Congo red staining fail to distinguish bacterial cells from the biofilm matrix [10].
This study introduces a novel dual-staining method combining Maneval’s stain and Congo red stain to visualize and differentiate microbial biofilms. Maneval’s stain, initially developed by Edmond Maneval in 1941 for visualizing bacterial capsules, has been adapted in this study for biofilm detection [11]. This simple, cost-effective method requires only basic equipment and minimal reagents, making it suitable for routine laboratory use. The dual-staining method effectively differentiates bacterial cells and biofilm matrix, displaying a distinctive blue polysaccharide layer surrounding the magenta-red bacterial cells. Congo red interacts with the hydrophobic regions of polysaccharides through hydrogen bonds [12], initially staining them red. Upon adding Maneval’s stain, the pH becomes acidic, causing a shift to blue due to the protonation of the azo group [13]. Simultaneously, acid fuchsin binds to the negatively charged bacterial surfaces, creating a magenta-red coloration.
We applied this technique to various microbial species, including Gram-positive bacteria with a thick peptidoglycan layer (Staphylococcus aureus and Enterococcus faecalis), Gram-negative bacteria distinguished by their outer membrane (Escherichia coli and Pseudomonas aeruginosa), and fungi (Candida albicans), which differ significantly in the presence of chitin, glucan, and mannoproteins. While this method does not provide the detailed structural resolution of high-magnification techniques like SEM, it offers a practical and accessible alternative for distinguishing biofilm matrix and bacterial cells.
Materials and reagents
Biological materials
1. Microbial culture (any bacterial or fungal culture including clinical isolates or standard strains such as ATCC)
Reagents
1. 1% Congo red (HIMEDIA Laboratories, catalog number: GRM927)
2. Maneval’s stain (Carolina Biological Supply, catalog number: 873785 or prepared in-house, see Recipe 2)
3. 4% Formaldehyde
Caution: Formaldehyde is a hazardous chemical and should be handled in a well-ventilated area with appropriate personal protective equipment (PPE), including gloves, lab coat, and safety goggles.
4. Nutrient broth (HIMEDIA Laboratories, catalog number: M002) or any basic broth (e.g., LB broth, TSB broth, BHI broth)
Solutions
1. 1% Congo red solution (see Recipes)
2. Maneval’s stain (see Recipes)
Recipes
1. 1% Congo red solution
| Reagent | Quantity or Volume |
|---|---|
| Congo red | 1 g |
| Distilled water | 100 mL |
2. Maneval’s stain [14]
| Reagent | Quantity or Volume |
|---|---|
| Fuchsin | 0.05 g |
| Ferric chloride | 3.0 g |
| Acetic acid | 5 mL |
| Phenol | 3.9 mL |
| Distilled water | 95 mL |
Laboratory supplies
1. Glass slides [Blue Star (PIC1), 1.35 mm thick, 75 mm× 25 mm]
2. Petri plates (SRL Pvt. Ltd., polystyrene 90 mm × 15 mm, catalog number: 90226)
3. Droppers (Plastic Labware India, catalog number: PPPD101)
4. Staining rack (Advance Scientific & Surgical Co., catalog number: ADV-188)
5. Microscope oil (cedarwood oil) (SRL Pvt. Ltd., catalog number: 31018)
6. Gloves (Microgene Diagnostic Systems Pvt. Ltd.)
Equipment
1. Light microscope (Radical Scientific Equipment Pvt. Ltd., model: (RXL-5 Binocular Research Microscope)
2. Incubator (set to 37 °C)
Procedure
文章信息
稿件历史记录
提交日期: Dec 12, 2024
接收日期: Jan 31, 2025
在线发布日期: Feb 24, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Nirmala, B. and Omar, B. J. (2025). Microbial Biofilm Detection and Differentiation by Dual Staining Using Maneval’s Stain. Bio-protocol 15(5): e5228. DOI: 10.21769/BioProtoc.5228.
- B, N., Manhas, P. L., Jadli, M., Sharma, R., Manhas, H. and Omar, B. J. (2024). A novel dual-staining method for cost-effective visualization and differentiation of microbial biofilms. Sci Rep. 14(1): 29169. https://doi.org/10.1038/s41598-024-80644-3
分类
微生物学 > 微生物生物膜 > 生物膜检测
微生物学 > 微生物细胞生物学 > 细胞染色
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link