- EN - English
- CN - 中文
Isolation and Biophysical Characterization of Extracellular Vesicles From Hairy Root Cultures
毛状根培养物中细胞外囊泡的分离与生物物理特性分析
(*contributed equally to this work) 发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5225 浏览次数: 2218
评审: Noelia ForesiAnonymous reviewer(s)
Abstract
Extracellular vesicles (EVs) are membrane-bound, non-replicating particles released by virtually all types of cells. EVs concentrate and deliver a plethora of biomolecules driving very important biological functions, including intercellular communication not only between cells of the same organism but also across different kingdoms. Plant extracellular vesicles (PEVs) are a promising alternative to mammalian EVs in biomedical applications. Here, we present an optimized and reproducible protocol for isolating PEVs from the hairy root (HR) cultures of medicinal plants Salvia dominica and S. sclarea. Our methodological approach introduces a significant advancement in the standardization of HR-EVs purification processes from plant biotechnological platforms, paving the way for their broader application across various sectors, including agriculture, pharmaceuticals, and nutraceuticals.
Key features
• Hairy roots serve as a plant biotechnological platform for the production of bioactive compounds.
• Plant extracellular vesicles (EVs) provide a safer alternative to mammalian EVs for biomedical applications.
• This protocol represents a significant advancement in the standardization of plant EV purification.
Keywords: Plant biotechnological platforms (植物生物技术平台)Graphical overview
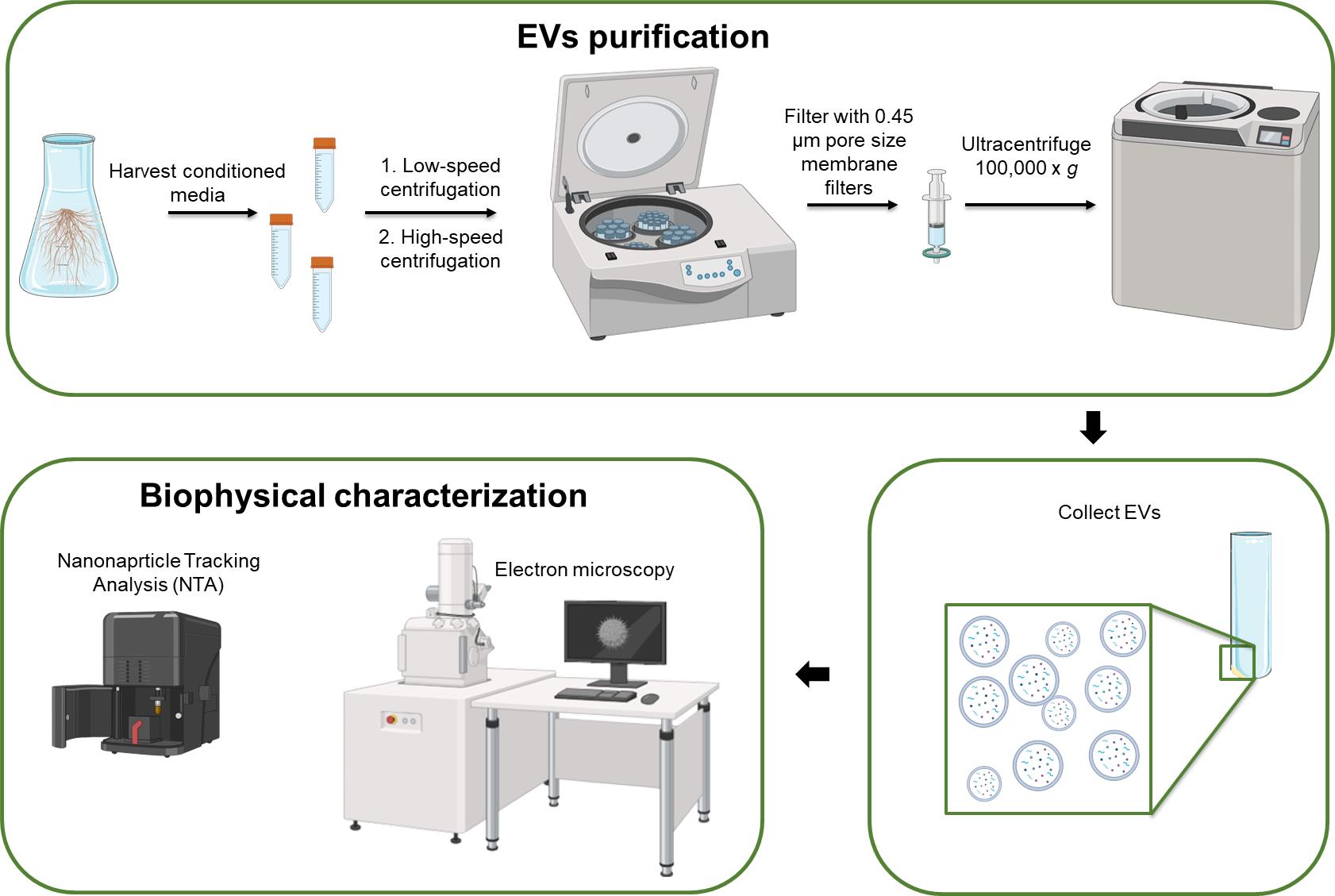
Background
Extracellular vesicles (EVs) are membrane-bound, nano- to micro-sized particles released by cells in various physiological and pathological settings. These vesicles transport proteins, lipids, nucleic acids, and metabolites, facilitating the exchange of molecular information and influencing biological functions in recipient cells [1]. EVs play a critical role in establishing complex networks of intercellular communication, extending their impact across different species and even between biological kingdoms [2]. Owing to their intrinsic features, such as cargo bioactivity and efficient cell uptake, EVs hold significant potential for therapeutic applications. However, the use of mammalian-derived EVs in therapeutic development faces significant challenges, including high production costs, complex isolation protocols, and safety concerns related to immune responses and reproducibility [3]. These limitations have driven interest in plant extracellular vesicles (PEVs), which offer a safer, more sustainable, and cost-effective solution [4,5]. PEVs are non-toxic, demonstrate minimal immunogenicity, and are less likely to provoke adverse reactions in humans, making them a valuable alternative to mammalian EVs in biomedical applications [4]. Interestingly, PEVs carry a wide variety of bioactive molecules that support tissue regeneration and exhibit anti-cancer and anti-inflammatory properties [6,7].
However, PEVs are usually obtained from plant tissues and organs using destructive extraction methods. As a result, the preparations often contain a mixture of EVs alongside other vesicles, including intracellular and artificially generated nano- and microvesicles. Furthermore, isolating EVs from fresh fruits and vegetables poses challenges in standardizing EV preparations, as they can vary significantly between batches due to factors such as genetic diversity within the species, seasonal variations, agricultural treatments, soil composition, and geographical origin.
To address these limitations, we recently adopted hairy root (HR) cultures as biotechnological biofactories for EV production [8,9]. Generated through Agrobacterium rhizogenes–mediated transformation, HRs have a long history of being used to produce high-value bioactive compounds [10]. HR cultures grow continuously under controlled conditions without the need for hormones, ensuring the consistent quality of HR-EVs, a peculiar type of PEV, which have shown interesting therapeutic bioactivity. HRs also provide economic and environmental advantages, as they require minimal inputs and sustainable growth conditions and do not rely on animal-derived materials. Their scalability further enhances their appeal as a cost-effective solution for both clinical and industrial EV production [11].
Herein, we present a robust and reproducible procedure for purifying and characterizing EVs from the conditioned media of HR cultures of the medicinal plants Salvia dominica and Salvia sclarea. This methodological approach, initially developed by Boccia et al. [8] and later refined by Vestuto et al. [9], can be adapted for isolating PEVs from other HR cultures and plant cell suspension cultures.
Materials and reagents
Biological materials
1. Salvia sclarea seeds (Lot # 2494) were purchased from B&T World Seeds, France
2. Salvia dominica seeds were kindly provided by Prof. Ammar Bader (Faculty of Pharmacy Umm Al-Qura University Makkah, Saudi Arabia). A voucher specimen number JO/IT-2022/1 has been deposited in the Herbarium of Laboratory of Pharmacognosy at Umm Al-Qura University, Makkah, Saudi Arabia
3. Agrobacterium rhizogenes virulent strain (ATCC 15834)
Reagents
1. Ethanol (Carlo Erba, catalog number: 414608)
2. Milli-Q water
3. Sodium hypochlorite solution (Supelco, catalog number: 1.05614)
4. Murashige & Skoog salt mixture including vitamins (Duchefa, catalog number: M0221)
5. Microagar (Duchefa, catalog number: M1002)
6. Yeast extract (AppliChem, catalog number: A1553)
7. Beef extract (Sigma-Aldrich, catalog number: B4888)
8. Peptone (AppliChem, catalog number: A2210)
9. Sucrose (Thermo Scientific, catalog number: A15583.0C)
10. MgSO4 (AppliChem, catalog number: A6414)
11. Cefotaxime (Sigma-Aldrich, catalog number: C7039)
12. NaCl (HiMedia, catalog number: GRM031)
13. KCl (Sigma-Aldrich, catalog number: P9333)
14. Na2HPO4 (Sigma-Aldrich, catalog number: S3264)
15. KH2PO4 (Sigma-Aldrich, catalog number: P5655)
16. NaOH (AppliChem, catalog number: A3910)
17. HCl (Sigma-Aldrich, catalog number: 30721-M)
18. Phire Plant Direct PCR kit (Thermo Fischer Scientific, catalog number: F130WH)
19. Agarose (Euroclone, catalog number: EMR900)
20. SYBR Safe DNA gel stain (Thermo Fisher Scientific, catalog number: S33102)
21. EDTA (Sigma-Aldrich, catalog number: ED2SS)
22. Sodium dodecyl sulfate (SDS) (SAFC, catalog number: 8.17034)
23. 30% Acrylamide/Bis Solution, 29:1 (Bio-Rad, catalog number: 1610156)
24. Tris-HCl (Sigma-Aldrich, catalog number: T3253)
25. Ammonium persulfate (APS) (Bio-Rad, catalog number: 1610700)
26. N,N,N',N'-Tetramethyl ethylenediamine (TEMED) (Sigma-Aldrich, catalog number: T2, 250-0)
27. Glycine (Sigma-Aldrich, catalog number: G8898)
28. Trizma base (Invitrogen, catalog number: 15504-20)
29. DTT (Dithiothreitol) (Thermo Fischer Scientific, catalog number: R0861) or β-mercaptoethanol (Sigma-Aldrich, catalog number: M3148)
30. Bromophenol blue (AppliChem, catalog number: A2331)
31. Methanol (Carlo Erba, catalog number: 414816)
32. Glacial acetic acid (Sigma-Aldrich, catalog number: 71251)
33. Sodium thiosulfate (Sigma-Aldrich, catalog number: 7772-98-7)
34. AgNO3 (Thermo Fisher, catalog number: 197680050)
35. Sodium carbonate (J.T. Baker, catalog number: 3602-01)
36. Paraformaldehyde (Sigma-Aldrich, catalog number: 158127)
37. Formaldehyde (Sigma-Aldrich, catalog number: F8775)
38. Glutaraldehyde solution (Sigma-Aldrich, catalog number: 354400)
39. Methylamine Vanadate (NanoVan) (Nanoprobes, catalog number: NC0659245)
40. Primers (5'→3'):
rolB (Forward, Fw) GATTCAACCATATCGGAGCG
rolB (Reverse, Rv) TGAGCATGTGCTGTTTTTGG
rolC (Fw) CGACCTGTGTTCTCTTTTTCAAGC
rolC (Rv) GCACTCGCCATGCCTCACCAACTCACC
virD2 (Fw) ATGCCCGATCGAGCTCAAGT
virD2 (Rv) CCTGACCCAAACATCTCGGCT
Actin (Fw) GGTGCCCTGAGGTCCTGTT
Actin (Rv) GAGCCACCACTGAGGACAAT
Solutions
1. YEB medium (see Recipes)
2. MS30 medium (see Recipes)
3. 50× TAE (see Recipes)
4. 10× PBS (see Recipes)
5. 10% resolving gel (see Recipes)
6. 4% stacking gel (see Recipes)
7. 4× Laemmli buffer (see Recipes)
8. 10× Tris-glycine-SDS (TGS) Buffer (see Recipes)
9. Fixing solution (see Recipes)
10. Washing 1 (see Recipes)
11. Sensitizing solution (see Recipes)
12. Silver solution (see Recipes)
13. Developing solution (see Recipes)
Recipes
1. YEB medium
Yeast extract 0.1%
Beef extract 0.5%
Peptone 0.5%
Sucrose 0.5%
MgSO4 0.04%
Autoclave and store at 4 °C.
Prepare with distilled water.
2. MS30 medium
Murashige & Skoog salt mixture + vitamins 4.3 g/L
Sucrose 30 g/L
Cefotaxime 100 mg/L or 50 mg/L
Microagar 9 g/L (for solid media only)
Prepare with distilled water. Adjust pH to 5.8 and autoclave. Antibiotics should be added after autoclaving when the medium has cooled down to 40–50 °C to avoid degradation. Use a pre-prepared antibiotic stock solution (e.g., 50 mg/mL or 100 mg/L) for accurate dosing. Once prepared, store the medium at 4 °C for optimal stability.
To prepare MS30 solid medium, add 9 g/L of Microagar to MS30 before autoclaving. After autoclaving, allow the medium to cool down to 40–50 °C before adding the antibiotic. Then, immediately pour the medium into 100 mm × 15 mm Petri dishes under a sterile hood. Leave the dishes uncovered until the medium is completely solidified to prevent condensation. Once solidified, cover the plates and ensure they are sealed appropriately. The medium can be stored at 4 °C for up to two weeks.
3. 50× TAE
EDTA disodium salt 50 mM
Trizma base 2 M
Glacial/acetic acid 1 M
Prepare with Milli-Q water.
4. 10× PBS
NaCl 1.37 M
KCl 27 mM
Na2HPO4 100 mM
KH2PO4 18 mM
Adjust pH to 7.4, autoclave, and store at room temperature.
Prepare with Milli-Q water.
5. 10% resolving gel
Tris-HCl (pH 8.8) 375 mM
SDS 0.1%
Acrylamide 10%
TEMED 0.01%
APS 0.1%
Prepare with Milli-Q water.
6. 4% stacking gel
Tris-HCl (pH 6.8) 125 mM
SDS 0.1%
Acrylamide 4%
TEMED 0.01%
APS 0.1%
Prepare with Milli-Q water.
7. 4× Laemmli buffer
Tris-HCl (pH 6.8) 1.0 M
SDS 40% (w/v)
DTT or β-mercaptoethanol 0.2 M
Bromophenol blue 0.8% (w/v)
Prepare with Milli-Q water.
8. 10× TGS buffer
Trizma base 0.25 M
Glycine 1.92 M
SDS 1%
Prepare with Milli-Q water.
9. Fixing solution
Methanol 50%
Acetic acid 12%
Prepare fresh with Milli-Q water.
10. Washing 1
Methanol 50%
Prepare fresh with Milli-Q water.
11. Sensitizing solution
Sodium thiosulfate 0.04%
Prepare fresh with Milli-Q water.
12. Silver solution
Silver nitrate (AgNO3) 0.1%
Prepare fresh, keep in the dark, and use Milli-Q water.
13. Developing solution
Sodium carbonate 3%
Formaldehyde 0.05%
Prepare fresh with Milli-Q water.
Laboratory supplies
1. Micropipettes (Gilson, catalog number: F167360)
2. Pipette tips (Sarstedt, catalog numbers: 70.3010.100, 70.3030.200, 70.3050.205)
3. DeckWorks low binding tips (Corning, catalog numbers: 4150, 4148, 4149)
4. 50 mL conical tubes (Euroclone, catalog number: CC430828)
5. 1.5 mL microcentrifuge tubes (Euroclone, catalog number: ET3415)
6. Petri dishes (Thermo Fisher Scientific, catalog number: 172931)
7. Borosilicate glass jars (VWR, catalog number: 214-1132)
8. Tweezers (VWR, catalog number: OUTI0-AXAL)
9. Scalpels (VWR, catalog number: 233-0047)
10. Mortar and pestle (VWR, catalog numbers: 410-0112, 410-0119)
11. Syringes (Euroclone, catalog number: LC11352017R)
12. 0.45 μm pore size membrane filters (Euroclone, catalog number: EPSPE4530)
13. Carbon SEM stubs (Agar Scientific, catalog number: AGG399F)
Equipment
1. Autoclave (PB International)
2. Sterile laminar flow hood (HeraguardTM)
3. pH meter (XS instruments)
4. Grow chamber
5. Spectrophotometer (Thermo Fischer Scientific, model: Multiskan spectrum)
6. PCR thermocycler (Applied Biosystem, model: GeneAmpTM PCR System 9700)
7. Microcentrifuge (Eppendorf, model: 5415R)
8. Orbital shaker incubator (ZetaLab, model: Universal table shaker 709)
9. Ultracentrifuge (Beckman Coulter, model: OptimaTM L-90K)
10. Beckman Type 70 Ti fixed angle titanium rotor (Beckman Coulter)
11. Polypropylene open-top ultracentrifuge tubes (Beckman Coulter, catalog number: 326823)
12. -80 °C fridge (Thermo Fischer Scientific)
13. Mini-PROTEAN Tetra Vertical Electrophoresis Cell (Bio-Rad)
14. PowerPac Universal power (Bio-Rad)
15. NanoSight NS300 Instrument (Marvel Panalytical)
16. Scanning electron microscope (SEM) (Tescan Solaris)
17. Transmission electron microscope (TEM) (FEI Tecnai T20)
Software and datasets
1. EM-MENU software
2. NanoSight NTA 3.4 software
3. Mascot software (v2.5, Matrix Science)
4. Zetasizer software (v7.01, Malvern Instrument)
5. BioRender (https://www.biorender.com/) and SMART - Servier Medical ART (https://smart.servier.com/). The following figures were created using BioRender: Graphical overview, https://BioRender.com/g49p506; Figure 2, https://BioRender.com/l06m694.
Procedure
文章信息
稿件历史记录
提交日期: Nov 1, 2024
接收日期: Jan 20, 2025
在线发布日期: Feb 16, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Conte, M., Cappetta, E., Alfieri, M., Bifolco, M., Boccia, E., Vietri, M. and Ambrosone, A. (2025). Isolation and Biophysical Characterization of Extracellular Vesicles From Hairy Root Cultures. Bio-protocol 15(5): e5225. DOI: 10.21769/BioProtoc.5225.
分类
植物科学 > 植物细胞生物学 > 细胞器分离
生物科学 > 生物技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














