- EN - English
- CN - 中文
Voltage Clamp Fluorometry in Xenopus laevis Oocytes to Study the Voltage-sensing Phosphatase
电压钳荧光法研究非洲爪蟾卵母细胞中的电压感应磷酸酶
发布: 2025年02月20日第15卷第4期 DOI: 10.21769/BioProtoc.5212 浏览次数: 1990
评审: Willy R Carrasquel-UrsulaezAkira KarasawaXiaochen SunVinaykumar Idikuda

相关实验方案
高灵敏且可调控的 ATOM 荧光生物传感器:用于检测细胞中蛋白质靶点的亚细胞定位
Harsimranjit Sekhon [...] Stewart N. Loh
2025年03月20日 2241 阅读
Abstract
Voltage clamp fluorometry (VCF) is a powerful technique in which the voltage of a cell’s membrane is clamped to control voltage-sensitive membrane proteins while simultaneously measuring fluorescent signals from a protein of interest. By combining fluorescence measurements with electrophysiology, VCF provides real-time measurement of a protein’s motions, which gives insight into its function. This protocol describes the use of VCF to study a membrane protein, the voltage-sensing phosphatase (VSP). VSP is a 3 and 5 phosphatidylinositol phosphate (PIP) phosphatase coupled to a voltage sensing domain (VSD). The VSD of VSP is homologous to the VSD of ion channels, with four transmembrane helices (S1–S4). The S4 contains the gating charge arginine residues that sense the membrane’s electric field. Membrane depolarization moves the S4 into a state that activates the cytosolic phosphatase domain. To monitor the movement of S4, the environmentally sensitive fluorophore tetramethylrhodamine-6-maleimide (TMRM) is attached extracellularly to the S3-S4 loop. Using VCF, the resulting fluorescence signals from the S4 movement measure the kinetics of activation and repolarization, as well as the voltage dependence of the VSD. This protocol details the steps to express VSP in Xenopus laevis oocytes and then acquire and analyze the resulting VCF data. VCF is advantageous as it provides voltage control of VSP in a native membrane while quantitatively assessing the functional properties of the VSD.
Key features
• Voltage clamp fluorometry using Xenopus laevis oocytes expressing the voltage-sensing phosphatase of Ciona intestinalis.
• This protocol uses the fluorophore tetramethylrhodamine-6-maleimide (TMRM).
• This protocol details the procedure for a two-electrode voltage clamp using the Dagan CA-1B amplifier.
Keywords: Voltage clamp fluorometry (VCF) (电压钳荧光法(VCF))Graphical overview
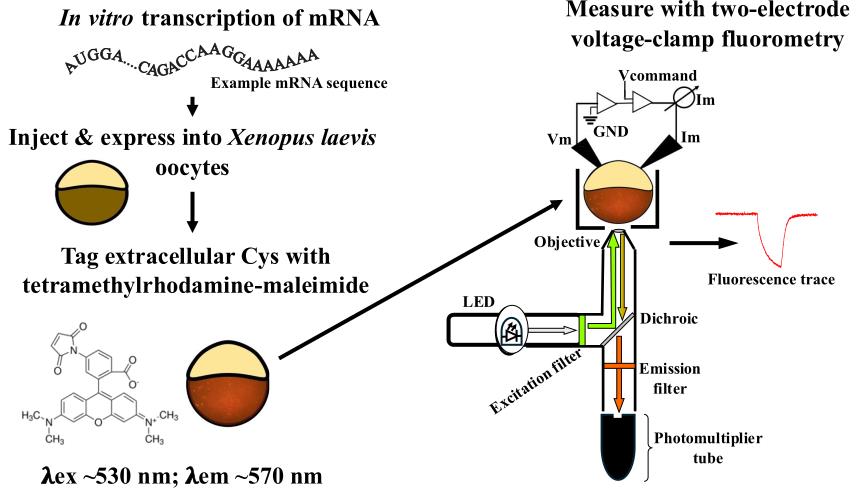
Background
Voltage clamp fluorometry (VCF) is a powerful technique to study voltage-sensitive conformational dynamics in membrane proteins. By combining the membrane voltage clamp with the measurement of fluorescent signals from the fluorophore attached to the protein of interest, real-time measurements of the protein’s conformational changes can be obtained and correlated to the electrophysiological functions. Initially developed to measure the conformational changes occurring in ion channels [1,2], the technique evolved to study other membrane proteins such as ion transporters [3,4] and, as discussed in this protocol, the voltage-sensing phosphatase (VSP) [5,6].
VSP is a 3 and 5 phosphatidylinositol phosphate (PIP) phosphatase coupled to a voltage-sensing domain (VSD) [7]. The VSD is made up of four transmembrane helices (S1–S4), with the S4 containing arginine residues that act as gating charges to sense the electric field of the membrane. Depolarization of the membrane causes the S4 to move from a down “resting” state in the membrane to an up “active” state. Unlike ion channels, the movement of the VSD in VSP does not lead to ionic currents; therefore, VCF is particularly advantageous as a robust measure of VSP’s voltage sensitivity and protein motions.
Environmentally sensitive fluorophores, like tetramethylrhodamine-6-maleimide (TMRM), are often used to monitor protein movements when attached to a protein’s predicted mobile region. Because the maleimide of TMRM is thiol-reactive, single cysteine mutations are introduced into these regions for labeling and are empirically tested for fluorescence changes that correlate with protein activation. To monitor S4 movements in VSP, G214C, at the top of S4 in the extracellular loop between S3 and S4, is a common labeling site. By analyzing the G214C-TMRM fluorescence signal during activation and repolarization conditions, the kinetics of the VSD, specifically S4, are calculated, giving us insight into the VSD motions in response to the changing voltage stimulus. Additionally, by taking the amplitude of the fluorescence signal relative to the membrane voltage, we calculate the voltage dependence of the VSD, which correlates with the voltage dependence of VSP’s phosphatase activity. Further, VCF labeling sites can be combined with other mutations to probe the mechanism of voltage dependence [6,8], coupling properties of the linker [9–11] and phosphatase activity [12,13]. While we only discuss the G214C labeling site here, different labeling sites will report on different protein motions and can be used to further test the mechanism of protein function [10,11].
Because Xenopus laevis (X. laevis) oocytes are large single cells that are easy to manipulate and maintain, they are a great expression system for VCF. This protocol describes all the different parts of preparing, setting up, and conducting a VCF experiment. It starts with how to prepare X. laevis oocytes from the ovary; it then describes how to make the messenger RNA (mRNA). It moves on to how to inject the oocytes with the mRNA for expression, how to label them with TMRM, and ultimately how to voltage clamp them using a Dagan CA-1B amplifier for two-electrode voltage clamp. Lastly, this protocol describes the steps for analyzing the acquired fluorescence signals.
Materials and reagents
Biological materials
1. Xenopus laevis ovaries (Xenopus One, catalog number: 10004, ¼ ovary)
2. Ciona intestinalis voltage-sensing phosphatase (Ci-VSP) in pSD64TF vector (Y. Okamura, Osaka University, Osaka, Japan, Addgene plasmid #80332) (Figure 1A)
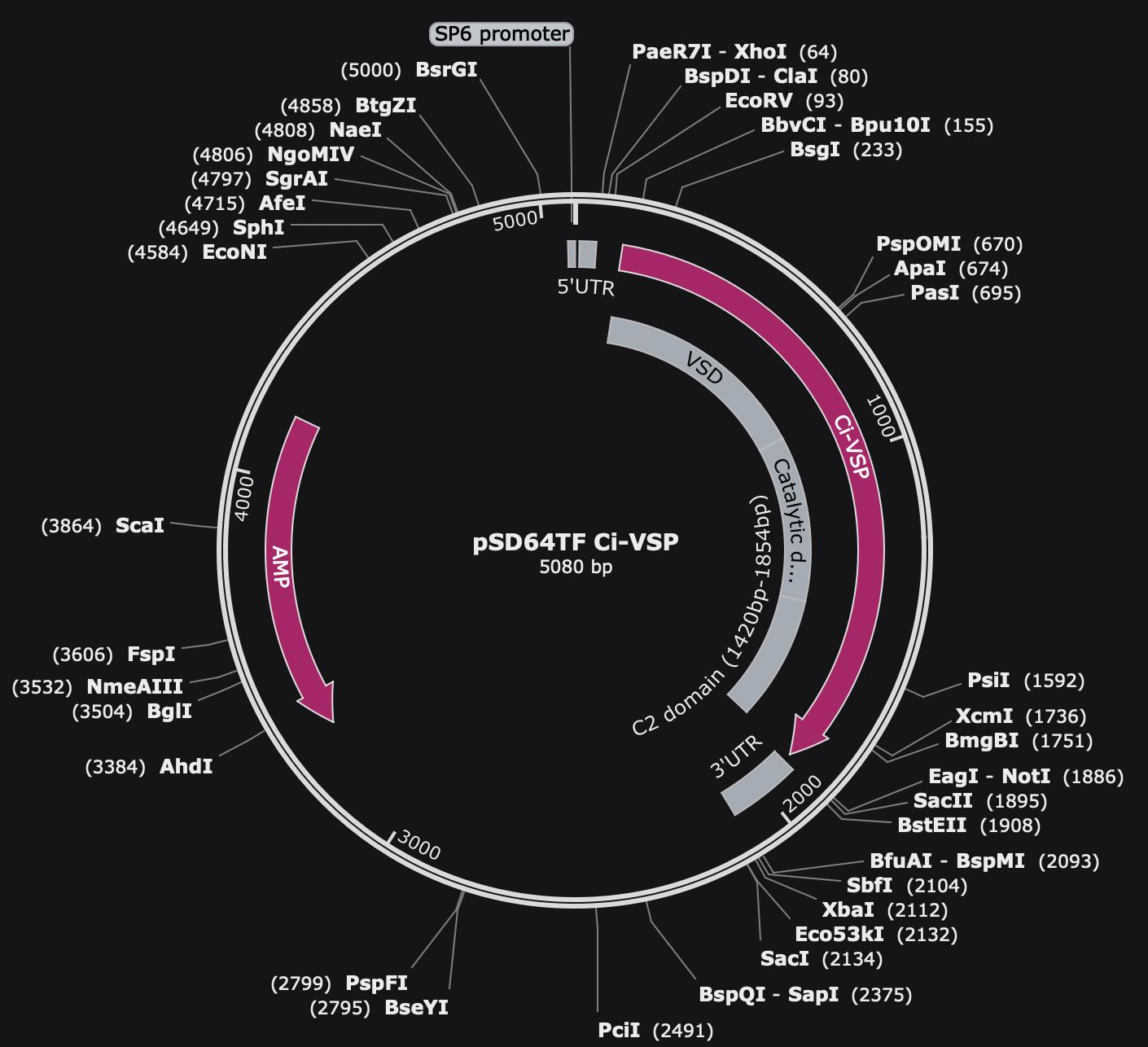
Figure 1. Map of pSD64TF Ci-VSP plasmid. Vector of pSD64TF with ampicillin selection and an SP6 promoter. The VSD, catalytic domain, and C2 domain of Ci-VSP are noted in the gray regions.
Reagents
1. mMESSAGE mMACHINE SP6 Transcription kit (Invitrogen, catalog number: AM1340)
2. Linearization enzyme XbaI (NEB, catalog number: R3131L)
3. Water, DNase/RNase free (Fisher, catalog number: BP561-1)
4. Ethyl alcohol 200 proof (EtOH) (Pharmco, catalog number: 111000200)
5. Ethyl alcohol 190 proof (EtOH) (Pharmco, catalog number: 111000190)
6. GeneRuler DNA ladder (Thermo Scientific, catalog number: SM0333)
7. Millennium RNA marker (Thermo Fisher, catalog number: AM7150)
8. QIAquick PCR Purification kit (QIAGEN, catalog number: 28104)
9. Light mineral oil (Millipore Sigma, catalog number: ES005C)
10. Collagenase type 2 (Worthington Biochemical Corporation, catalog number: LS004177)
11. Collagenase type 3 (Worthington Biochemical Corporation, catalog number: LS004183)
12. Tetramethylrhodamine-6-maleimide (TMRM) (Abcam, catalog number: ab145471)
13. Sodium pyruvate (Fisher, catalog number: BP356-100)
14. NaCl (Fisher, catalog number: BP358-1)
15. KCl (Fisher, catalog number: BP366-500)
16. CaCl2·2H2O (JT Baker, catalog number: 1332-01)
17. MgCl2·6H2O (Thermo Scientific, catalog number: J62575.36)
18. Gentamicin sulfate (Thermo Scientific, catalog number: J6283406)
19. HEPES (Fisher, catalog number: BP310-500)
20. N,N-Dimethylformamide (DMF) (Thermo Scientific, catalog number: 041859AK)
21. HCl (Fisher, catalog number: S25358)
22. NaOH (Fisher, catalog number: P250-500)
23. RNase Away (Thermo Scientific, catalog number: 7005-11)
Solutions
1. ND-96 (see Recipes)
2. Ca2+-free buffer (see Recipes)
3. ND-96(-) (see Recipes)
4. Bridge buffer (see Recipes)
Recipes
1. ND-96 (1 L)
Note: Adjust pH to 7.6 with HCl or NaOH and filter-sterilize to prevent contaminating growth. Store at room temperature for up to a year or longer as long as it does not show any growth.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 96 mM | 56.1 g |
| KCl | 2 mM | 0.15 g |
| CaCl2·2H2O | 1.8 mM | 0.265 g |
| MgCl2·6H2O | 1 mM | 0.203 g |
| Gentamicin sulfate | 50 μg/mL | 2 mL of 50 mg/mL stock |
| Sodium pyruvate | 2.5 mM | 5 mL of 1 M stock |
| HEPES | 10 mM | 2.383 g |
| Total | n/a | 1,000 mL |
2. Ca2+-free buffer (1 L)
Note: Adjust pH to 7.6 with HCl or NaOH and autoclave to sterilize. Store at room temperature for up to a year or longer as long as it does not show any growth.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 83 mM | 4.8 g |
| KCl | 2 mM | 0.15 g |
| MgCl2·6H2O | 1 mM | 0.203 g |
| HEPES | 10 mM | 2.383 g |
| Total | n/a | 1,000 mL |
3. ND-96(-) (1 L)
Note: Adjust pH to 7.6 with HCl or NaOH and autoclave to sterilize. Store at room temperature for up to a year or longer as long as it does not show any growth.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 96 mM | 56.1 g |
| KCl | 2 mM | 0.15 g |
| CaCl2·2H2O | 1.8 mM | 0.265 g |
| MgCl2·6H2O | 1 mM | 0.203 g |
| HEPES | 10 mM | 2.383 g |
| Total | n/a | 1,000 mL |
4. Bridge buffer (1 L)
Note: Adjust pH to 7.4 with HCl or NaOH and autoclave to sterilize. Store at room temperature for up to a year or longer as long as it does not show any growth.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 1 M | 58.4 g |
| HEPES | 10 mM | 2.383 g |
| Total | n/a | 1,000 mL |
Laboratory supplies
1. Reusable 45 mm bottle top filter (Nalgene, catalog number: DS0320-5045)
2. Membrane and prefilter disks (Nalgene, catalog number: DS02154020)
3. Low-retention filtered RNase-free pipette tips (Fisher, catalog numbers: 02-707-002, 02-707-006, and 02-707-008)
4. 3.5” glass capillaries (Drummond Scientific, catalog number: 3-000-203-G/X)
5. Glass capillary tubes (VWR, catalog number: 5432-921)
6. Glass coverslips, No. 1, 22 × 40 mm (Warner Instruments, catalog number: 64-0707)
7. Polypropylene microcentrifuge tubes (Globe Scientific, catalog number: 11563)
8. Falcon Petri dishes (Falcon, catalog number: 351007)
9. 50 mL conical tubes (Thermo Fisher, catalog number: AM12502)
10. 25 G × 5/8 in. needle (BD, catalog number: 305122)
11. 1 mL syringe (BD, catalog number: 309628)
12. DrieriteTM 10–20 mesh (Thermo Scientific, catalog number: 219065000)
13. 150 mm × 15 mm Petri dish (Falcon, catalog number: 351058)
14. Palladium wire (Thermo Scientific, catalog number: AA45072G1)
Equipment
1. Belly Dancer Orbital Shaker (IBI Scientific, model: BDRAA115S)
2. Glass Pasteur pipette (Fisher, catalog number: 13-678-4A)
3. Blunted glass Pasteur pipette (homemade using the pipettes in #2)
4. Mini low-temperature refrigerated incubator, 18 L (Fisher, catalog number: 15-015-2632)
5. Water bath 2 L digital (Benchmark Scientific, catalog number: B2000-2)
6. Refrigerated high-speed microcentrifuge (Thomas Scientific, catalog number: 1154Q52)
7. Rotor (Thomas Scientific, model: AS-24-2)
8. Centrifugal vacuum concentrator (Thermo Scientific, model: DNA120)
9. NanoDropTM One spectrophotometer (Thermo Scientific, catalog number: ND-ONE-W)
10. Nanoject II Auto-Nanoliter Injector (Drummond Scientific, catalog number: 3-000-204)
11. Dissecting microscope (Olympus, model: SZ61)
12. Manipulator (Märzhäuser Wetzlar, catalog number: 00-42-101-0000)
13. Flaming/Brown micropipette puller (Sutter Instruments, model: P-97)
14. Inverted microscope (Leica, model: DM IRBE, catalog number: 020-525.701 to 020-525.780)
15. HC Pl APO 20×/0.7 fluorescence objective (Leica, catalog number: 506166)
16. Amplifier (Dagan Corporation, model: CA-1B)
17. Photomultiplier tube (PMT) (ThorLabs, catalog number: PMTSS2)
18. Axon Digidata-1440A (Molecular Devices Instruments, catalog number: DD1440A)
19. X-Cite XLED1 light source (Lumen Dynamics, catalog number: 010-00288R)
20. LED 505-546 nm (Lumen Dynamics, model: BGX)
21. Cy3 Leica cube set with HQ531/40 excitation filter, HQ593/40 emission filter, Q562LP dichroic (Semrock, catalog number: Cy3-4040C-LSC-ZERO)
22. Eight-pole Bessel filter (Frequency Devices, model: 900CT)
23. Gravity glass puller (Narishige, model: PC-10)
24. Vibration isolation platform (TMC, catalog number: 77049189)
25. Faraday cages (homemade)
26. Glass agarose bridges (homemade)
27. Bath chamber (Warner Instruments, model: RC-24E)
28. Bath chamber platform (Warner Instruments, model: 64-1526)
29. Inox tweezers style #5 (Dumont, catalog number: 11254-20)
30. Mesh 0.011 diameter, 18 × 16 mesh count (Phifer, catalog number: 3002201)
Software and datasets
1. pClamp version 10.3 software package (Axon Instruments)
2. Microsoft Excel, version 16.9 for Mac (Microsoft)
3. Igor Pro version 8 software (WaveMetrics)
Procedure
文章信息
稿件历史记录
提交日期: Nov 11, 2024
接收日期: Dec 30, 2024
在线发布日期: Jan 19, 2025
出版日期: Feb 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Young, V. C., Rayaprolu, V. and Kohout, S. C. (2025). Voltage Clamp Fluorometry in Xenopus laevis Oocytes to Study the Voltage-sensing Phosphatase. Bio-protocol 15(4): e5212. DOI: 10.21769/BioProtoc.5212.
分类
生物物理学 > 电生理 > 膜片钳技术
生物化学 > 蛋白质 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link