- EN - English
- CN - 中文
Versatile Click Chemistry-based Approaches to Illuminate DNA and RNA G-Quadruplexes in Human Cells
基于多功能点击化学的人细胞内DNA/RNA G-四链体成像方法
发布: 2025年02月05日第15卷第3期 DOI: 10.21769/BioProtoc.5209 浏览次数: 2548
评审: Emilie BesnardAnonymous reviewer(s)
Abstract
The existence and functional relevance of DNA and RNA G-quadruplexes (G4s) in human cells is now beyond debate, but how did we reach such a level of confidence? Thanks to a panoply of molecular tools and techniques that are now routinely implemented in wet labs. Among them, G4 imaging ranks high because of its reliability and practical convenience, which now makes cellular G4 detection quick and easy; also, because this technique is sensitive and responsive to any G4 modulations in cells, which thus allows gaining precious insights into G4 biology. Herein, we briefly explain what a G4 is and how they can be visualized in human cells; then, we present the strategy we have been developing for several years now for in situ click G4 imaging, which relies on the use of biomimetic G4 ligands referred to as TASQs (for template-assembled synthetic G-quartets) and is far more straightforward and modular than classically used immunodetection methods. We thus show why and how to illuminate G4s with TASQs and provide a detailed, step-by-step methodology (including the preparation of the materials, the methodology per se, and a series of notes to address any possible pitfalls that may arise during the experiments) to make G4 imaging ever easier to operate.
Key features
• MultiTASQs are clickable probes usable for the detection of cellular DNA and RNA G-quadruplex (G4).
• In situ click chemistry relies on the labeling of G4 by clickable probes once in their cellular binding sites.
• Experiments can be performed by incubating the clickable probe either in live cells or in fixed cells.
• The published but unoptimized protocol is now totally revised to allow for reliable G4 detection in human cells.
Keywords: DNA/RNA G-quadruplex (G4) (DNA/RNA G-四链体(G4))Graphical overview
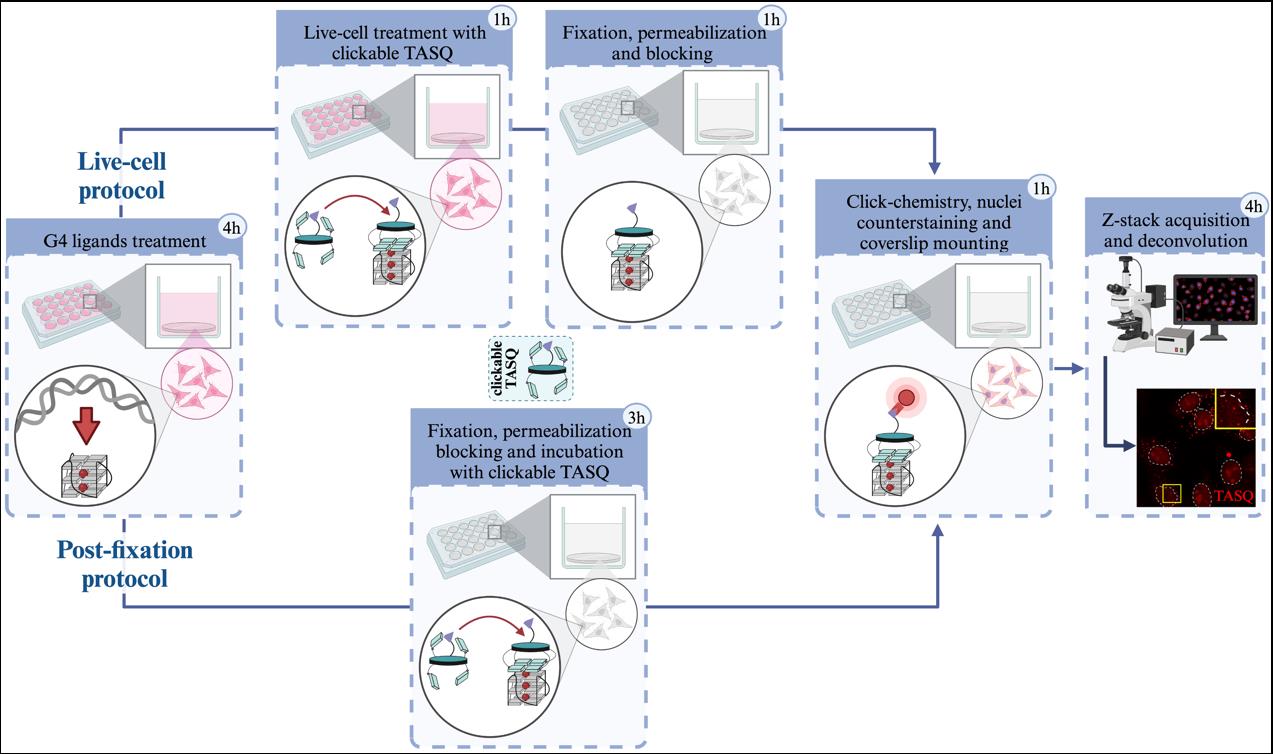
Background
Guanine (G)-rich DNA and RNA sequences are being studied for their ability to fold into G-quadruplex (G4) structures [1–3]. G4-prone sequences are widespread in our genome and transcriptome; it has now been established that G4s are key players in virtually all genomic transactions, from the regulation of genetic and epigenetic events to chromatin organization [1–8]. G4-prone sequences have been identified in silico by various methods [9], which showed that >1 million sequences can fold into G4s in our genome [10] and transcriptome [11] (referred to as G4s and rG4s for DNA and RNA G4s, respectively). This formation has been demonstrated at both genome-wide and transcriptome-wide scales using methods being classified as in vitro (that is, using purified DNA and RNA samples: G4-seq [12] and G4DP-seq [13] for G4s, rG4-seq [14] for rG4s) or in vivo (that is, in a functional cellular context: G4 ChIP-seq [15], G4 CUT&Tag [16], and G4access [17] for G4s, G4RP-seq [18] for rG4s). These sequencing-based methods confirmed the prevalence of G4 landscapes in human cells, with the identification of hundreds of thousands in vitro G4s and tenths of thousands in vivo G4s [19].
The cellular roles of G4s are beyond the scope of the present article; interested readers are invited to consult recent reviews on this topic (for G4s, see [1,2,7]; for rG4s, see [3–6,8]). We would like to report here on a convergent strategy we have been developing for several years now, based on the use of smart molecular tools named template-assembled synthetic G-quartets (TASQs) (Figure 1) [20]. Our aims are to i) select the best-suited TASQs among those now available as a function of the intended application and ii) use this tool for addressing more than one chemical biology challenge (optical imaging, affinity purification, chemoproteomics, etc.). TASQs are structurally dynamic molecules comprising a central template surrounded by four flexible arms ending with a G unit; they are thus biomimetic ligands that stabilize G4s by interacting between two G-quartets, one from the G4, and one from the TASQ. We developed the smart PNADOTASQ [21], twice-as-smart PyroTASQ [22] and NaphthoTASQ (or N-TASQ) [23], biotinylated BioTASQ [24], BioCyTASQ [25] and BioTriazoTASQ [26], and multivalent MultiTASQ and azMultiTASQ [27]. These TASQs fold into their G4-affinic closed conformation only upon interaction with G4s (that is, only when a G-rich sequence folds into a G4 structure), which makes them uniquely actively selective for their G4 targets [20,21].
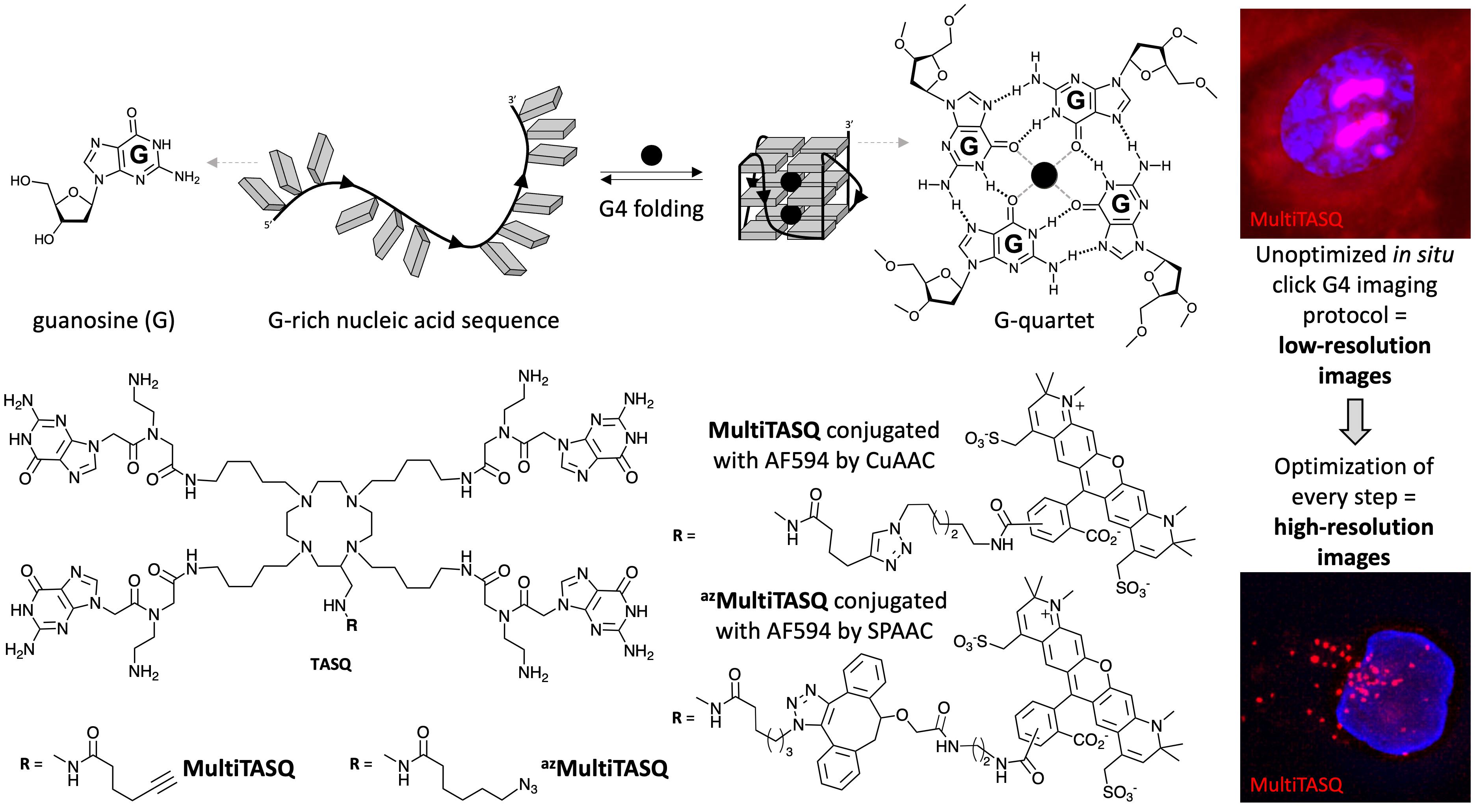
Figure 1. Schematic representation of the folding of a G-quadruplex (G4) structure from a guanine (G)-rich DNA sequence. Chemical structure of TASQs, either MultiTASQ or azMultiTASQ, and of their derivatives after click chemistry (CuAAC or SPAAC)-based coupling with a fluorophore; the resulting conjugate aimed at being used to visualize G4s in human cells.
Here, we report on the use of MultiTASQs to illuminate G4s, given that MultiTASQ and azMultiTASQ are the sole clickable TASQs from this family of compounds. Several strategies have been pursued to visualize G4s in cells [28–30], but we are particularly interested in a quite efficient two-step methodology based on the fluorescent tagging of chemical ligands once in their genomic binding site by in situ click chemistry [31]. This yet indirect approach allows for better control of the fluorescence readout, which makes it more versatile. This approach, pioneered in the G4 field by Rodriguez et al. in 2012 [32], is here extended using now commercially available clickable TASQs. We thus provide all necessary technical details for performing in situ click G4 imaging using MultiTASQs, focusing on the step-by-step methodology and all possible cell manipulations to increase the quality of the collected images and all necessary controls to exploit the obtained results in a reliable manner.
Materials and reagents
Note: Caution must be exercised when performing TASQs staining experiments; the most important features are gathered in the different notes below (see General notes 1–4), which must be read before preparing the materials needed for the experiments. All solutions must be prepared (unless otherwise stated) in ultrapure water (resistivity ≥ 18 MΩ·cm at 25 °C), and molecular biology and/or analytical grade reagents must be used. Solutions can be prepared and stored at room temperature (unless otherwise stated); some of them need to be prepared under a sterile environment in the biological safety cabinet (BSC) and/or to be filtered (PES 0.22 μm bottle-top vacuum filter system or syringe filter system, depending on volume).
Biological materials
1. HeLa cell line (ATCC, catalog number: CCL-2)
Reagents
Note: As not all reagents and/or solutions are absolutely required (see General notes 1–4) and some are mutually exclusive, we strongly recommend reading the procedure in its entirety to determine if they should be used.
Note: We provide the list of reagents we used, but reagents from alternative suppliers are acceptable as long as they are molecular biology and/or analytical-grade reagents.
1. Dulbecco’s modified Eagle medium (DMEM) containing sodium pyruvate, high glucose, and GlutaMAX (Gibco, catalog number: 31966021); store at 4 °C
2. Fetal bovine serum (FBS) 100% (Dutscher, catalog number: on request); store at -80 °C
3. Penicillin/streptomycin 10,000 U/mL (Gibco, catalog number: 15140122); store at -20 °C
4. Trypsin-EDTA (0.25%), phenol red (Gibco, catalog number: 25200056); once thawed, store at 4 °C
5. PBS 10× (Eurobio, catalog number: ET330A)
6. Pyridostatin hydrochloride (PDS) (Sigma-Aldrich, catalog number: SML2690-5MG)
7. Dulbecco’s PBS 10× (DPBS) (Dutscher, catalog number: X0515-500)
8. TBS 10× (Euromedex, catalog number: ET220-B)
9. Triton X-100 (Sigma-Aldrich, catalog number: X100-100ML); store at 4 °C
10. Tween-20 (Fisher BioReagents, catalog number: BP337-500)
11. 1,4-Piperazinediethanesulfonic acid (PIPES) (Sigma-Aldrich, catalog number: P8203)
12. Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: 71376-1KG)
13. Sucrose (Sigma-Aldrich, catalog number: S0389)
14. Magnesium chloride (MgCl2) (Sigma-Aldrich, catalog number: M8266)
15. RNase A 10 mg/mL (Thermo Scientific, catalog number: EN0531); store at -20 °C
16. TURBOTM DNase kit 2 U/μL containing TURBOTM DNase and 10× TURBOTM DNase buffer (Invitrogen, catalog number: AM2238); store at -20 °C
17. Methanol (MeOH) (Carlo Erba Reagents, catalog number: 412383); store at -20 °C
18. Paraformaldehyde (PFA) 16% w/v aqueous solution, methanol free (Thermo Scientific, catalog number: 28908); once opened, store in a 15 mL Falcon tube at 4 °C
19. Bovine serum albumin (BSA) (Seqens, catalog number: 1000-70); store at 4 °C
20. Fish gelatin (Sigma-Aldrich, catalog number: G7041); store at 4 °C
21. MultiTASQ (Merck, catalog number: SCT247); store at -20 °C
22. azMultiTASQ (not commercially available, contact D. Monchaud); store at -20 °C
23. Alexa FluorTM 594 azide (AF-az) 1 mM in DMSO [Alexa FluorTM 594 carboxamido-(6-azidohexanyl), triethylammonium salt], mixed isomers (Invitrogen, catalog number: A10270); store at -20 °C, avoid light
24. Click-iTTM Alexa FluorTM 594 sDIBO alkyne (DIBO-AF) 5 mM in DMSO (Invitrogen, catalog number: C10407); store at -20 °C, avoid light
25. Copper(II) sulfate (CuSO4) (Sigma-Aldrich, catalog number: C7631-250G)
26. IGEPAL CA-630 (Sigma-Aldrich, catalog number: I8896)
27. Sodium ascorbate (NaAsc) (Sigma-Aldrich, catalog number: 11140)
28. 4',6-Diamidino-2-phenylindole dihydrochloride (DAPI) (Sigma-Aldrich, catalog number: D9542)
29. VECTASHIELD® antifade mounting medium (VectorLab, catalog number: H1900); store at 4 °C
30. Transparent nail polish
Solutions
Note: Filtered solutions must be aliquoted to avoid cross-contamination and/or bacterial counterstaining with DNA intercalating dyes. Solutions with adjusted pH must be prepared with a sufficient volume of ultrapure water (ca. 90% of final volume) to resuspend powder by mixing, then pH must be adjusted, and finally, the solution can be completed with ultrapure water to final volume.
1. PDS 20 mM in DMSO
Note: Please refer to the manufacturer’s certificate of analysis to obtain the molecular weight of PDS with the number (y) of molecules of HCl as counterion and with the number (z) of molecules of water [final molecular weight (g/mol) = 596.64 + y × 36.46 + z × 18.02]. Store at -80 °C.
2. MultiTASQ or azMultiTASQ 10 mM in nuclease-free water
Note: Molecular weight of MultiTASQ: 1801.12 g/mol. Molecular weight of azMultiTASQ: 1846.16 g/mol. To avoid batch-to-batch variations in concentrations of stock solution of TASQ, we recommend measuring the absorbance of TASQ’s guanines at λ = 246 nm (see section A. Preparation of TASQ). Store at -20 °C.
3. AF-az 1 mM in DMSO
Note: Please refer to the manufacturer’s molecular weight depending on the number of triethylammonium molecules as counterion (molecular weight should be around 1,050 g/mol for 2 counterions). Store at -20 °C, avoid light.
4. DIBO-AF 5 mM in DMSO
Note: Please refer to the manufacturer’s molecular weight depending on the number of triethylammonium molecules as counterion (molecular weight should be around 1,200 g/mol for 2 counterions). Store at -20 °C, avoid light. Intermediate dilution of 0.5 mM of DIBO-AF in water (10% final concentration of DMSO) is more handful for SPAAC mix.
5. DAPI 1 mg/mL in water
Note: Store at 4 °C, avoid light.
6. Sterile PBS 1× (see Recipes)
7. Sterile supplemented DMEM (see Recipes)
8. Filtered DPBS 1× (see Recipes)
9. Filtered DPBS-Tx (see Recipes)
10. Filtered TBS-Tw (see Recipes)
11. PIPES 100 mM, pH 7.0 (see Recipes)
12. NaCl 1 M (see Recipes)
13. MgCl2 1 M (see Recipes)
14. Filtered sucrose 1 M (see Recipes)
15. Filtered cytoskeletal pre-extraction (CSK) buffer (see Recipes)
16. Filtered BSA blocking buffer (see Recipes)
17. Filtered FBS blocking buffer (see Recipes)
18. MultiTASQ 20 μM (200 μL) for post-fixation staining (see Recipes)
19. azMultiTASQ 20 μM (200 μL) for post-fixation staining (see Recipes)
20. CuSO4 100 mM (see Recipes)
21. IGEPAL CA-630 10% (see Recipes)
22. NaAsc 300 mg/mL (150×) (see Recipes)
23. CuAAC mix (see Recipes)
24. SPAAC mix (see Recipes)
Recipes
1. Sterile PBS 1× (500 mL)
Note: Prepare for cell culture (sterilize by filtration or autoclaving) and open only under sterile conditions.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS (10×) | 1× | 50 mL |
| H2O | n/a | 450 mL |
| Total | n/a | 500 mL |
2. Sterile supplemented DMEM (555 mL)
Note: Prepare directly in a DMEM bottle (from the manufacturer) and open only under sterile conditions.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DMEM (1×) | n/a | 500 mL |
| FBS (100%) | 10% | 55 mL |
| Penicillin/Streptomycin (100%) | 1% | 5.5 mL |
| Total | n/a | ca. 555 mL |
3. Filtered DPBS 1× (500 mL)
Note: Filter and aliquot 50 mL for use. Keep a 50 mL aliquot at 4 °C as cold DPBS.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DPBS (10×) | 1× | 50 mL |
| H2O | n/a | 450 mL |
| Total | n/a | 500 mL |
4. Filtered DPBS-Tx (500 mL)
Note: Filter and aliquot 50 mL for use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DPBS (10×) | 1× | 50 mL |
| H2O | n/a | 450 mL |
| Triton X-100 (100%) | 0.1% | 500 μL* |
| Total | n/a | ca. 500 mL |
*Tip needs to be cut due to Triton X-100 viscosity.
5. Filtered TBS-Tw (500 mL)
Note: Filter and aliquot 50 mL for use.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| TBS (10×) | 1× | 50 mL |
| H2O | n/a | 450 mL |
| Tween-20 (100%) | 0.1% | 500 μL* |
| Total | n/a | ca. 500 mL |
*Tip needs to be cut due to Tween-20 viscosity.
6. PIPES 100 mM, pH 7.0 (50 mL)
Note: Store at 4 °C up to a year. We recommend controlling pH if PIPES has not been used in the past 3 months.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PIPES | 100 mM | 1.51 g |
| H2O | n/a | n/a* |
| NaOH 10 M | n/a | Until reaching pH 7.0** |
| Total | n/a | 50 mL |
*Add 40 mL of ultrapure water before adjusting pH. Once pH is adjusted, complete with water to obtain 50 mL of solution.
**pH equilibration with NaOH 10 M is necessary to dissolve PIPES; adjust pH to 7.0 by adding dropwise NaOH in solution under stirring.
7. NaCl 1 M (50 mL)
Note: Store at 4 °C for up to a year.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 1 M | 2.92 g |
| H2O | n/a | n/a* |
| Total | n/a | 50 mL |
*Add 40 mL of ultrapure water and allow the solution to mix completely before adding any more water.
8. MgCl2 1 M (50 mL)
Note: Store at 4 °C for up to a year, avoid light.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| MgCl2 | 1 M | 4.76 g |
| H2O | n/a | n/a* |
| Total | n/a | 50 mL |
*Add 40 mL of ultrapure water and allow the solution to mix completely before adding any more water.
9. Filtered sucrose 1 M (50 mL)
Note: Filter and store at -20 °C to avoid contamination.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Sucrose | 1 M | 17.1 g |
| H2O | n/a | n/a* |
| Total | n/a | 50 mL |
*Add 30 mL of ultrapure water and allow the solution to mix completely before adding any more water as sucrose will take a substantial volume when fully dissolved.
10. Filtered cytoskeletal pre-extraction (CSK) buffer (50 mL)
Note: Filter with a 0.22 μm PES filter for a syringe and aliquot 5–10 mL of CSK in vials under sterile conditions. Store aliquoted vials at -20 °C. Avoid freeze/thaw cycles of aliquoted vials (2–3 cycles maximum).
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PIPES (100 mM, pH 7.0) | 10 mM | 5 mL |
| NaCl (1 M) | 100 mM | 5 mL |
| Sucrose (1 M) | 300 mM | 15 mL |
| MgCl2 (1 M) | 3 mM | 150 μL |
| H2O | n/a | 24.5 mL |
| Triton X-100 (100 %) | 0.7% | 350 μL* |
| Total | n/a | 50 mL |
*Tip needs to be cut due to Triton X-100 viscosity. Homogenize before adding Triton X-100 to avoid foaming.
11. Filtered BSA blocking buffer (500 mL)
Note: We found that the appropriate blocking buffer for MultiTASQ is BSA blocking buffer, and that for azMultiTASQ is FBS blocking buffer (see Troubleshooting 4; see Figure 10).
Filter and aliquot 50 mL of blocking buffer in vials under sterile conditions. Store aliquoted vials at -20 °C. Once a vial is thawed, store at 4 °C for 2–3 months maximum.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| BSA | 1% (w/v) | 5 g |
| Fish gelatin | 0.2% (w/v) | 1 g |
| DPBS (10×) | 1× | 50 mL |
| H2O | n/a | n/a |
| Triton X-100 (100 %) | 0.1% | 500 μL* |
| Total | n/a | ca. 500 mL |
*Add 300 mL of water, allow BSA and fish gelatin to dissolve, and complete to 500 mL before addition of Triton X-100. The tip needs to be cut due to Triton X-100 viscosity. Homogenize before adding Triton X-100 to avoid foaming.
12. Filtered FBS blocking buffer (50 mL)
Note. We found that the appropriate blocking buffer for MultiTASQ is BSA blocking buffer, and that for azMultiTASQ is FBS blocking buffer (see Troubleshooting 4; see Figure 10).
Prepare and filter under sterile conditions with a 0.22 μm PES filter for a syringe. Store at 4 °C for 1–2 months maximum.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| FBS (100 %) | 2% | 1 mL |
| DPBS-Tx | n/a | 49 mL |
| Total | n/a | 50 mL |
13. MultiTASQ 20 μM (200 μL) for post-fixation staining
Note: Prepare just before incubation with MultiTASQ (see Procedure for post-fixation staining). This volume corresponds to the volume required for a well (see General note 5). This solution cannot be stored.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| MultiTASQ (2 mM, see section A) | 20 μM | 2 μL |
| Filtered BSA blocking buffer | n/a | 198 μL |
| Total | n/a | 200 μL |
14. azMultiTASQ 20 μM (200 μL) for post-fixation staining
Note: Prepare just before incubation with azMultiTASQ (see Procedure for post-fixation staining). This volume corresponds to the volume required for a well (see General note 5). This solution cannot be stored.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| azMultiTASQ (2 mM, see section A) | 20 μM | 2 μL |
| Filtered FBS blocking buffer | n/a | 198 μL |
| Total | n/a | 200 μL |
15. CuSO4 100 mM (1 mL)
Note: Store at 4 °C and avoid light.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CuSO4 | 100 mM | 24.97 mg |
| H2O | n/a | 1 mL |
| Total | n/a | ca. 1 mL |
16. IGEPAL CA-630 10% (1 mL)
Note: Store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| IGEPAL CA-630 (100%) | 10 % | 100 μL* |
| H2O | n/a | 900 μL |
| Total | n/a | 1 mL |
*Tip needs to be cut due to IGEPAL CA-630 viscosity.
17. NaAsc 300 mg/mL (150×) (300 μL)
Note: Aliquot vials of 30 μL and store at -20 °C. Avoid freeze/thaw cycle as NaAsc is sensitive to oxidation.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaAsc | 300 mg/mL | 90 mg |
| H2O | n/a | 300 μL |
| Total | n/a | ca. 300 μL |
18. CuAAC mix (200 μL)
Note: Prepare just before “click” chemistry step with MultiTASQ (see Procedure). This volume corresponds to the volume required for a well (see General note 5). Add reagents in the order listed below. This solution cannot be stored.
NaAsc 30 mg/mL (or 15×) should be prepared in nuclease-free ultra-pure water before CuAAC mix; for 15 μL, add 1.5 μL of NaAsc 300 mg/mL to 13.5 μL of nuclease-free ultra-pure water.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| PBS (1×) | n/a | 184 μL |
| IGEPAL (10%) | 0.05% | 1 μL |
| CuSO4 (100 mM) | 1 mM | 2 μL |
| AF-az (1 mM) | 1 μM | 0.2 μL |
| NaAsc (30 mg/mL or 15×) | 2 mg/mL or 1× | 13.3 μL |
| Total | n/a | ca. 200 μL |
19. SPAAC mix (200 μL)
Note: Prepare just before “click” chemistry step with azMultiTASQ (see Procedure). This volume corresponds to the volume required for a well (see General note 5). This solution cannot be stored.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| DIBO-AF (0.5 mM) | 0.5 μM | 0.2 μL |
| FBS blocking buffer (1×) | n/a | 200 μL |
| Total | n/a | ca. 200 μL |
Laboratory supplies
1. PES filter 0.22 μm Stericup system (Sigma-Aldrich, catalog number: S2GPU10RE)
2. OmnifixTM solo 50 mL single-use syringe (Braun, catalog number: 4613503F)
3. PES filter 0.2 μm (MinisartTM high flow) for syringe (Sartorius, catalog number: 16532----------K)
4. Tissue culture treated flask (75 cm2) (Falcon, catalog number: 353136)
5. Glass coverslips 13 mm, #1.5 (VWR, catalog number: 631-0150)
6. Microscope glass slides 26 × 76 × 1 mm (ISO8037/I) (Epredia, catalog number: AA00000112E01MNZ10)
7. 24-well polystyrene microplate (2 cm2) (Falcon, catalog number: 353047) or 4-well polystyrene microplate (2 cm2) (SPL Life Science, catalog number: 30004)
Equipment
1. pH meter
2. Spectrophotometer (UV5Nano)
3. Biological safety cabinet
4. 37 °C, 5% CO2 incubator
Note: Equipment is dedicated to mammalian cell culture to avoid any bacterial contamination.
5. Aspiration system
Note: The aspiration system allows hazardous biological fluids (cell debris, fixative reagent, DAPI, etc.) to be disposed of.
6. Counting chamber (Malassez pattern)
7. Phase-contrast or brightfield microscope
8. Widefield microscope (Olympus, model: IX83)
a. DAPI filter set (Chroma, catalog number 49000)
b. TexasRed filter set (Chroma, catalog number 49306)
c. Optional for multiplexing: GFP filter set (Chroma, catalog number 49002) and/or Cy5 filter set (Chroma, catalog number 49009) and/or Cy7 filter set (Chroma, catalog number 49007)
Software and datasets
1. CellSens Dimension (Olympus, v3.2)
2. FIJI (ImageJ, v2.14.0)
3. Excel (Microsoft, v16.77.1)
4. Prism (GraphPad, v10.3.1)
5. Code has been deposited to GitHub (see Data analysis)
Procedure
文章信息
稿件历史记录
提交日期: Oct 25, 2024
接收日期: Dec 18, 2024
在线发布日期: Jan 19, 2025
出版日期: Feb 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Pipier, A. and Monchaud, D. (2025). Versatile Click Chemistry-based Approaches to Illuminate DNA and RNA G-Quadruplexes in Human Cells. Bio-protocol 15(3): e5209. DOI: 10.21769/BioProtoc.5209.
分类
分子生物学 > DNA > DNA 标记
分子生物学 > RNA > RNA 标记
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













