- EN - English
- CN - 中文
Combined FLIM, Confocal Microscopy, and STED Nanoscopy for Live-Cell Imaging
结合FLIM、共聚焦显微镜和STED纳米显微镜用于活细胞成像
发布: 2025年02月20日第15卷第4期 DOI: 10.21769/BioProtoc.5202 浏览次数: 2456
评审: Ivonne SehringAnonymous reviewer(s)
Abstract
Time-lapse fluorescence microscopy is a relevant technique to visualize biological events in living samples. Maintaining cell survival by limiting light-induced cellular stress is challenging and requires protocol development and image acquisition optimization. Here, we provide a guide by considering the quartet sample, probe, instrument, and image processing to obtain appropriate resolutions and information for live cell fluorescence imaging. The pleural mesothelial cell line H28, an adherent cell line that is easy to seed, was used to develop innovative advanced light microscopy strategies. The chosen red and near-infrared probes, capable of passively penetrating through the cell plasma membrane, are particularly suitable because their stimulation from 600 to 800 nm induces less cytotoxicity. The labeling protocol describes the concentration, time, and incubation conditions of the probes and associated adjustments for multi-labeling. To limit phototoxicity, acquisition parameters in advanced confocal laser scanning microscopy with a white laser are determined. Light power must be adjusted and minimized at red wavelengths for reduced irradiance (including a 775 nm depletion laser for STED nanoscopy), in simultaneous mode with hybrid detectors and combined with the fast FLIM module. These excellent conditions allow us to follow cellular and intracellular dynamics for a few minutes to several hours while maintaining good spatial and temporal resolutions. Lifetime analysis in lifetime imaging (modification of the lifetime depending on environmental conditions), lifetime dye unmixing (separation with respect to the lifetime value for the spectrally closed dye), and lifetime denoising (improvement of image quality) provide flexibility for multiplexing experiments.
Key features
• Cell preservation after labeling with less cytotoxic red, near-infrared dye viable probes.
• Determination of lower but efficient probe concentration; adjust good balance between probes concentration and incubation time to achieve multi-labeling.
• Long time-lapse acquisition in advanced confocal microscopy with sensitive new-generation detectors.
• Confocal image combined with fast FLIM for multi-labeling with spectrally closed dyes, unmixed from lifetime values.
• Confocal-STED image acquisition combined with fast FLIM to improve signal-to-noise ratio.
Keywords: Live-cell imaging (活细胞成像)Graphical overview
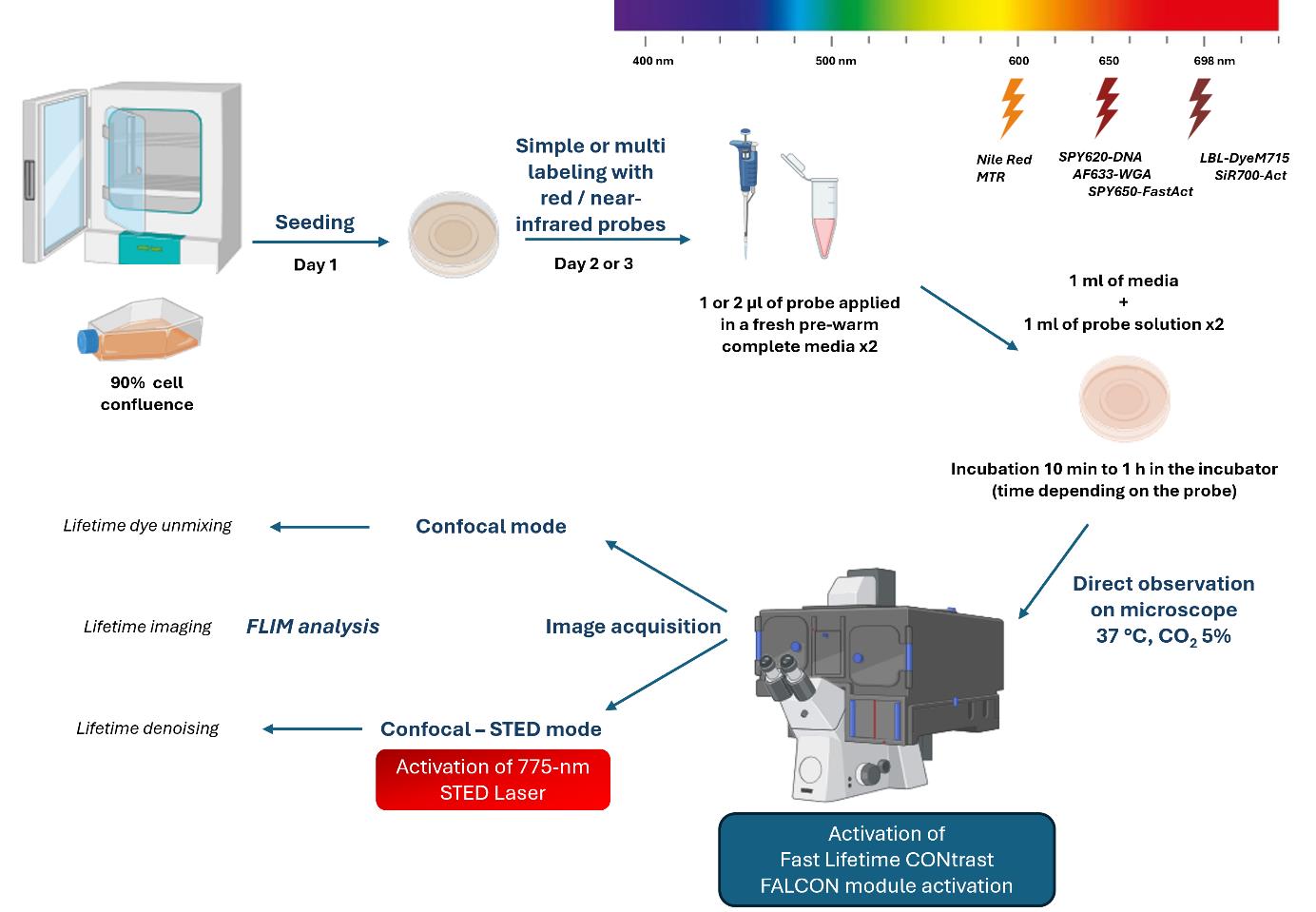
Background
Reducing cell exposure to study cellular dynamics is essential for cell biology research. As previously described [1], the quartet sample, probe, instrument, and image processing represent essential steps to conduct fluorescence microscopy experiments.
To preserve the viability of cells during imaging, the photophysical properties of dyes must be considered. In particular, their photostability under light excitation and brightness, with high quantic yield to reduce the light power to a minimum. The incorporation of the probe must be specific, efficient, and non-toxic inside the cell. The protocol must be optimized to determine the minimum effective concentration to strongly label the cell elements by limiting background noise. For multi-labeling, the concentration and incubation time balance must be optimized [2]. The choice of red/near-infrared dyes is compatible with living cells (red excitation light is less irradiating than lower wavelength illumination). The dye must be depletable with a 775 nm laser to perform STimulated-Emission-Depletion (STED) acquisition in nanoscopy.
To image cellular compartments, red fluorescent dyes linked to ubiquitous markers (e.g., wheat germ agglutinin Alexa Fluor 633 conjugate, AF633-WGA for lectin, Nile red for lipid, MitoTracker Red CMXRos, MTR and LBL-Dye M715 for mitochondria, SPY620-DNA for nucleus, SPY650-FastAct and SiR700-Act-Verapamil for actin cytoskeleton) were used for single and multiple labeling experiments. Their depletion capacity at 775 nm was verified and the lifetime characteristics (value and mono- or multi-components after exponential curve fitting) were determined [2,3].
To perform time-lapse multiplex acquisition by limiting light exposure, the dyes, all of which have a near-red wavelength, are stimulated and detected in one detector. Their specific lifetime values allow their separation in several fluorescence channels. The tunable white light laser (WLL) provides high flexibility in wavelength adjustment; the pulsed 775 nm laser depletes the red/near-infrared dyes in STED nanoscopy and induces less irradiance of living cells compared to other continuous depletion lasers [2,4]. The new generation of high-speed photon counting detectors, hybrid detectors (HyD), brought striking opportunities for high signal-to-noise ratios and fast detection of fluorescence signals, particularly true for HyD-X within the red/near-infrared range [2]. Biologist-friendly fluorescence lifetime imaging microscopy (FLIM), called fast FLIM [5–7], combined with confocal or confocal STED acquisition image, offers a flexible method suitable for cellular preservation and provides the lifetime parameter to xyzt dimensions while maintaining axial and temporal resolution.
Lifetime dye unmixing is an alternative to the limited fluorescence spectral separation. Simultaneous acquisition (one step), which is fast and limits cell light exposure compared to sequential acquisition, followed by lifetime dye unmixing allows (i) signal denoising by lifetime value selection to eliminate background or nonspecific signal, and (ii) dye fluorescence selection on the phasor, representing the photon lifetime distribution [8]. This method of multiplexing living cells can be achieved by activating one and eventually several detectors [9], which is more limited in STED nanoscopy. As the lifetime value can be influenced by factors such as viscosity, temperature, and pH [10–11], the lifetime imaging is informative about the cellular environment, revealing multi-components after fitting of the fluorescence decay curve; the resolution gain in STED microscopy can be improved in lifetime denoising modality by removing uncorrelated STED process photons and smoothing the signal. This protocol is applicable to all types of adherent cells and viable red/near-infrared dye probes.
Materials and reagents
Biological materials
1. Human H28 cell line (ATCC, NCI-H28, catalog number: CRL-5820) from the lungs of a 48-year-old white male with stage 4 mesothelioma.
Reagents
1. Roswell Park Memorial Institute (RPMI) 1640 cell culture medium (Thermo Fisher Scientific, Gibco, catalog number: 31870-025)
2. L-Glutamine 200 mM, 100× (Thermo Fisher Scientific, Gibco, catalog number: 25030-024)
3. Fetal bovine serum (FBS) (Life Technologies, Gibco, catalog number: 10270-106), heat-inactivated at 56 °C for 2 h
4. Antibiotic/antimycotic solution 100× with 10,000 U/mL penicillin G, 10,000 μg/mL streptomycin, and 25 μg/mL amphotericin B (HyClone, catalog number: SV30079.01)
5. Trypsin-EDTA with phenol red (0.25%) (Fisher Scientific, catalog number: 11560626)
6. Phosphate buffered saline (PBS), pH 7.4, 10× solution (Thermo Fisher Scientific, Gibco, catalog number: 70011-044)
7. Distilled water (Thermo Fisher Scientific, Invitrogen, catalog number: 10977-035)
8. Dimethyl sulfoxide (DMSO) (Corning, catalog number: 25-950-CQC)
9. Methanol anhydrous (Carlo Erba, catalog number: P09310D16)
10. Wheat germ agglutinin Alexa Fluor 633 conjugate (AF633-WGA) (Thermo Fisher Scientific, Invitrogen, catalog number: W21404)
11. Nile Red (Merck, Sigma-Aldrich, catalog number: N3013)
12. MitoTracker Red CMXRos (MTR) (Thermo Fisher Scientific, Life Technologies, catalog number: M7512)
13. LBL-DyeM 715 (Proimaging, catalog number: BM298)
14. SPY650-FastAct (Spirochrome, catalog number: SC505)
15. SPY620-DNA (Spirochrome, catalog number: SC404)
16. SiR700-Act and Verapamil kit (Spirochrome, catalog number: SC013)
Laboratory supplies
1. Cell culture flask 25 cm2, T-25 (TPP, catalog number: 90026)
2. 10 mL serological pipette (Merck, Costar, catalog number: CLS4488)
3. 5 mL serological pipette (Merck, Costar, catalog number: CLS4487)
4. 2 mL serological pipette (Merck, Costar, catalog number: CLS4486)
5. 15 mL Falcon tube (Falcon, catalog number: 352096)
6. 50 mL Falcon tube (Falcon, catalog number: 352070)
7. 1.5 mL microcentrifuge tubes (Eppendorf, catalog number: 022431081)
8. Glass-bottom microwell dishes, 35 mm Petri dish, 20 mm microwell, No. 1.5 coverglass 0.16–0.19 mm (MatTek Corporation, catalog number: P35G-1.5-20-C)
9. Neubauer chamber (EMS, catalog number: 68052)
10. Dropping bottles (Avantor, catalog number: HECH41314050)
Equipment
1. Class II biosafety cabinet (Thermo Scientific, catalog number: 51028226, model: MSC-ADVANTAGE)
2. CO2 incubator (Thermo Scientific, catalog number: 51032874, model: Heracell 150)
3. Heating oven (Thermo Scientific, catalog number: 51028112, model: Heratherm)
4. Safe liquid aspiration system Vacusafe (Avantor, Integra Biosciences, catalog number: 391-2094, model: Vacusafe Comfort plus)
5. Ultrasonicator (Ultrasonik Ney, model: 19H)
6. Pipette controller (Thermo Fisher Scientific, Integra Biosciences, catalog number: 155017, model: Pipetboy acu 2)
7. Mechanical pipette 0.1–2.5 μL (Eppendorf, catalog number: 3123000012, model: Research Plus)
8. Mechanical pipette 0.5–10 μL (Eppendorf, catalog number: 3123000020, model: Research Plus)
9. Mechanical pipette 20–200 μL (Eppendorf, catalog number: 3123000055, model: Research Plus)
10. Mechanical pipette 100–1000 μL (Eppendorf, catalog number: 3123000063, model: Research Plus)
11. Centrifuge for 15 mL and 50 mL tubes (Thermo Fisher Scientific, Eppendorf, catalog number: 5804000528, model: 5804R)
12. Microcentrifuge for tubes 1.5 mL (Thermo Fisher Scientific, Fisherbrand, catalog number: 75002460, model: Micro 17)
13. Power meter Argo-Power Slide (Argolight, model: Argo-POWER Slide V2)
14. Culture cell observation microscope (Leica Microsystems, model: DMIL LED)
15. Inverted stand confocal laser scanning microscope (Leica Microsystems, model: STELLARIS 8, FALCON STED 3 X WLL) equipped with a white light laser (WLL, 440–790 nm), 775 nm depletion laser for STED in living cells, four hybrid detectors (HyD type S, X, and R), an 86× objective (NA = 1.20, water immersion, WD = 300 µm), a fully fast integrated FLIM module FAst Lifetime CONtrast (FALCON, Leica Microsystems), and a full bold line Okolab chamber (Ottaviano, Italy) to keep the temperature at 37 °C and mix the CO2 at 5%
Software and datasets
1. Leica Application Suite X (LAS X, Leica Microsystems, version 4.5.0)
2. Leica Application Suite X FLIM/FCS (LAS X, Leica Microsystems, version 4.5.0)
3. Leica Application Suite X 3D viewer (Leica Microsystems, version 4.5.0)
4. Fiji/ImageJ (open source, National Institutes of Health, [12])
Procedure
文章信息
稿件历史记录
提交日期: Oct 4, 2024
接收日期: Dec 23, 2024
在线发布日期: Jan 9, 2025
出版日期: Feb 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Bénard, M., Chamot, C., Schapman, D., Lebon, A. and Galas, L. (2025). Combined FLIM, Confocal Microscopy, and STED Nanoscopy for Live-Cell Imaging. Bio-protocol 15(4): e5202. DOI: 10.21769/BioProtoc.5202.
分类
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link