- EN - English
- CN - 中文
Colorimetric Determination of Tungsten and Molybdenum in Biological Samples
生物样品中钨和钼的比色测定
发布: 2025年03月05日第15卷第5期 DOI: 10.21769/BioProtoc.5195 浏览次数: 1323
评审: Partha BasuKomuraiah MyakalaBhanu Jagilinki
Abstract
Molybdenum (Mo) and tungsten (W) are elements that are utilized in biological systems. They are typically incorporated into the catalytic sites of enzymes coordinated to an organic pyranopterin cofactor; Mo may also be present in the form of a FeMo cofactor. While Mo is used by all branches of life, only a few microbes are able to utilize W. In order to study Mo- and W-dependent enzymes, it is important to be able to measure Mo and W in biological samples. Methods for determining Mo and W content in biological samples currently involve expensive and time-consuming processes like inductively coupled plasma mass spectrometry (ICP-MS) and chelation ion chromatography. There are less intensive colorimetric methods for measuring W in abiotic samples, but these have not been adapted to biological samples like cytosolic extracts and purified proteins. Herein, we developed a colorimetric assay based on the complexation of quercetin to molybdate (MoO42-) or tungstate (WO42-), the oxyanion forms of Mo and W that readily form in denatured biological samples. In the assay, the absorbance of quercetin is redshifted proportionally to the concentration of tungsten or molybdenum, which can be measured spectrophotometrically. This protocol provides a rapid method for screening biological samples for both Mo and W, although it does not distinguish between them.
Key features
• This protocol is adapted from the method developed by El-Sayed et al. [1] for analyzing abiotic samples.
• The protocol is designed for a 96-well plate format and optimized for analyzing protein samples.
• Can be used over the range of 1–20 μM W or Mo.
• Allows for rapid and high throughput Mo and W determination in samples during protein purification.
Keywords: Tungsten (钨)Graphical overview
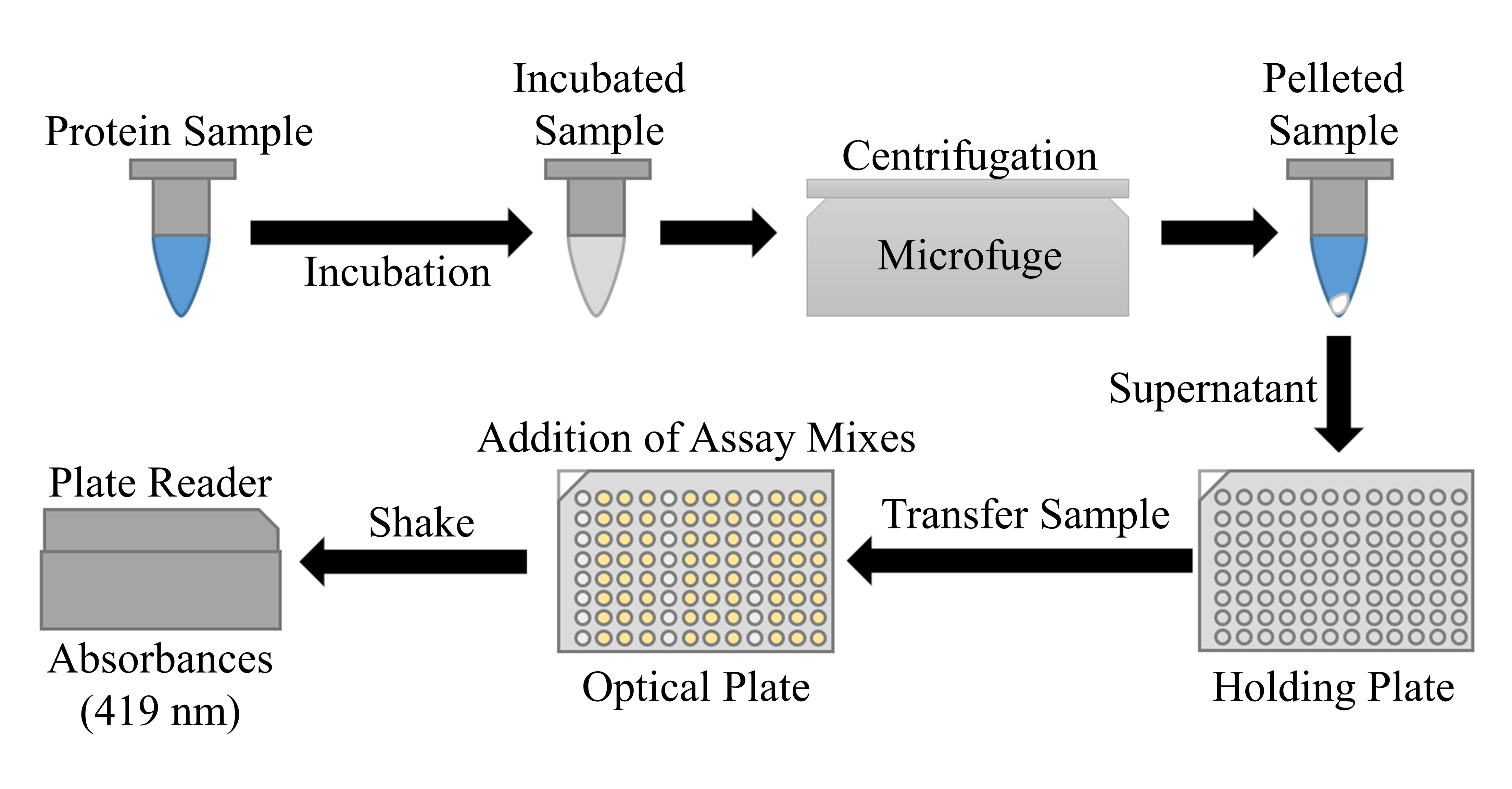
Overview of colorimetric determination of tungsten in protein samples using quercetin. Protein samples (200 μL) are transferred to microcentrifuge tubes along with 50 μL of 2.5× nitric acid mix and incubated at 65 °C overnight. The tubes are spun at 20,000× g for 20 min and 200 μL of the supernatant is transferred to a holding plate. 40 μL of each sample is transferred from the holding plate to four wells on an optical plate using a multichannel pipette. 160 μL of ethanol blank mix is added to one well per sample and 160 μL of quercetin mix is added to the remaining wells of each sample. The plate is sealed and shaken to mix each well. The seal is removed, and absorbances at 419 nm of each well are measured in a plate reader.
Background
Molybdenum (Mo) and tungsten (W) typically occur as pyranopterin cofactors at the catalytic sites of a variety of enzymes, except for Mo in the Mo-dependent nitrogenase, where it is part of a FeMo cofactor. Until now, methods for measuring these metals in biological samples were limited to resource-intensive protocols like high-performance liquid chromatography, ICP-MS, and voltammetry [2–4]. For the determination of Mo in the nitrogenase complex of Clostridium pasteurianum [5], a colorimetric assay was utilized, which involved the complexation and extraction of Mo with toluene-3,4-dithiol, determining Mo concentrations within the range of 0.2–100 μM [6]. However, this procedure is rather cumbersome and not readily adaptable to high throughput analysis. A simple colorimetric assay was developed for abiotic samples using a flavonol, quercetin, which complexes with free molybdate or tungstate in solution [1]. The formation of this complex is measured by its absorbance at 419 nm (Figure 1). In this report, the quercetin-based assay was adapted for biological samples, specifically protein samples from column chromatography fractions. Assay reagents and procedures were altered from the original assay to decrease interferences from substances present in biological samples, such as flavin and iron. To determine if the presence of protein, iron, and flavin in samples interfered with Mo and W measurements, standards were run with added protein (bovine serum albumin), iron (in the form of an iron-containing protein, a [4Fe-4S] ferredoxin), and flavin. As shown in Figures S1 and S2, the effects of these additions were small and not significant. The assay was also adapted to a 96-well plate format in order to simultaneously process large numbers of samples. Due to the chemical similarities between molybdate and tungstate, this assay can be utilized to determine the concentrations of either in biological samples but cannot distinguish between them [7].
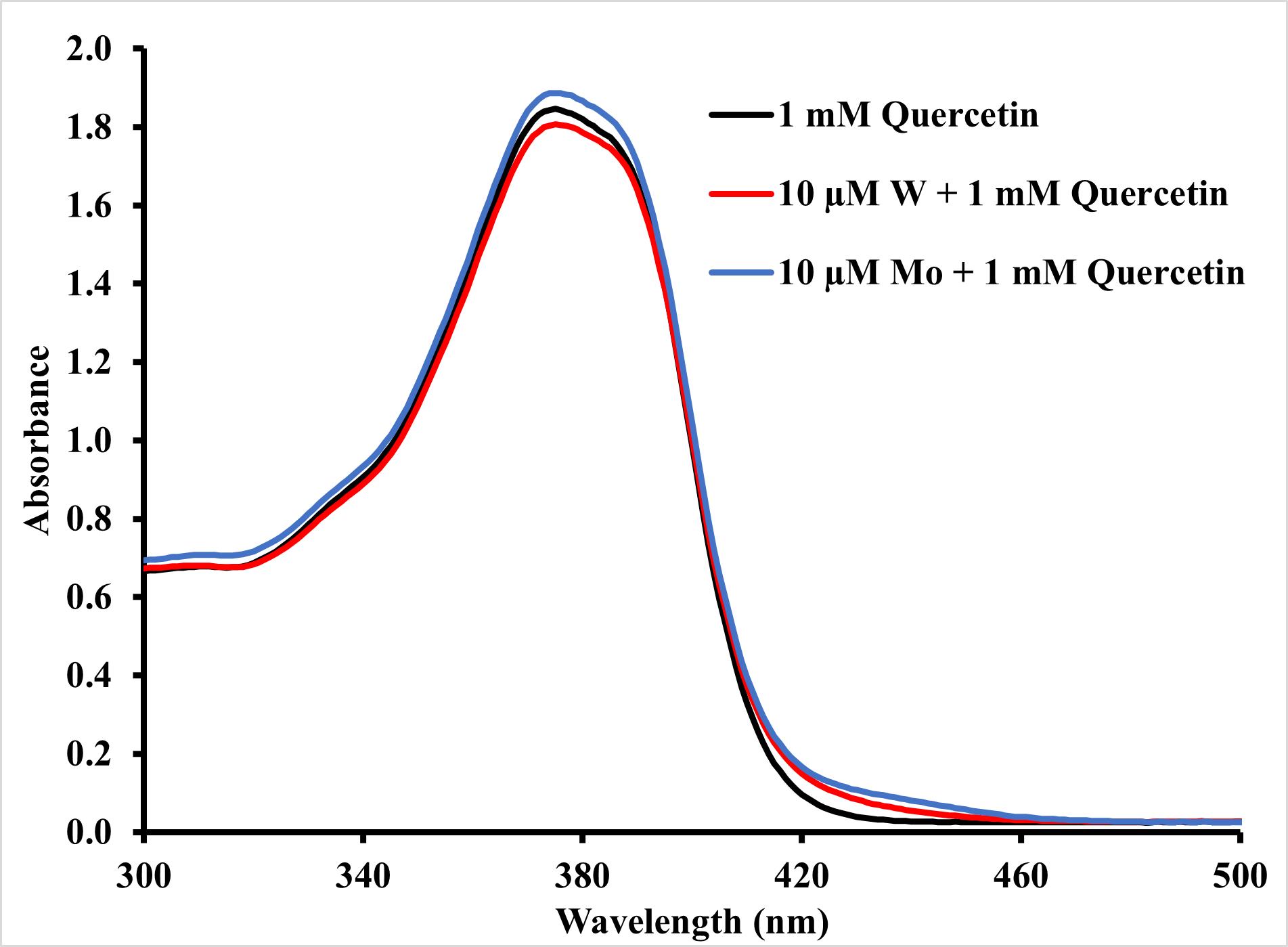
Figure 1. Effect of tungsten and molybdenum on quercetin spectra. Graph illustrating the change in spectra (300–500 nm) of 1 mM quercetin without W or Mo (black), with 10 μM W (red), and with 10 μM Mo (blue) blanked against ethanol.
Materials and reagents
Biological materials
1. Native tungsten-containing proteins in the cytosolic extract of the anaerobic bacterium Eubacterium limosum were separated anaerobically using anion exchange chromatography and further purified using size exclusion chromatography (SEC) by a Bio-Rad Enrich SEC 650 (10 × 300) column using 25 mM Tris-HCl, pH 7.6, and 100 mM NaCl buffer. These fractions contain the tungsten-binding protein, Tub, which is a hexamer of 7 kDa subunits and binds a total of eight tungsten oxyanions [8]. Sixteen column fractions from the SEC column were used in the tungsten assay.
Reagents
1. Cetylpyridinium chloride (CPC) (Sigma-Aldrich, catalog number: C0732)
2. Deionized water (diH2O)
3. Ethanol, absolute (Decon Laboratories, catalog number: 2716)
4. 70% nitric acid (Fisher Chemical, catalog number: EW-88050-70)
5. Quercetin hydrate (Sigma-Aldrich, catalog number: 337951)
6. Sodium fluoride (Sigma-Aldrich, catalog number: S1504)
7. Sodium molybdate dihydrate (Sigma-Aldrich, catalog number: M1651)
8. Sodium tungstate dihydrate (Sigma-Aldrich, catalog number: S0765)
Solutions
1. 1 mM quercetin in ethanol
2. 10 mM CPC
3. 1 M nitric acid
4. 0.1% sodium fluoride
5. 100 μM sodium tungstate
6. 100 μM sodium molybdate
7. 2.5× nitric acid mix (see Recipes)
8. 1× nitric acid mix (see Recipes)
9. Ethanol blank mix (see Recipes)
10. Quercetin assay mix (see Recipes)
Recipes
1. 2.5× nitric acid mix
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Nitric acid (1 M) | 250 mM | 25 mL |
| Sodium fluoride (0.1%) | 0.0025% | 2.5 mL |
| diH2O | n/a | 72.5 mL |
| Total | n/a | 100 mL |
2. 1× nitric acid mix
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Nitric acid (1 M) | 100 mM | 10 mL |
| Sodium fluoride (0.1%) | 0.001% | 1 mL |
| diH2O | n/a | 89 mL |
| Total | n/a | 100 mL |
3. Ethanol blank mix
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Ethanol (absolute) | 10% | 0.8 mL |
| Cetylpyridinium chloride (10 mM) | 2.5 mM | 2 mL |
| 1× nitric acid mix | n/a | 5.2 mL |
| Total | n/a | 8 mL |
4. Quercetin assay mix
*Note: Quercetin will begin to precipitate out of the solution more than 30 min after the formation of the mixture.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Quercetin (1 mM) in ethanol | 0.1 mM | 1.8 mL* |
| Cetylpyridinium chloride (10 mM) | 2.5 mM | 4.5 mL |
| 1× nitric acid mix | n/a | 11.7 mL |
| Total | n/a | 18 mL |
Laboratory supplies
1. 1.5 mL microcentrifuge tubes (Heathrow Scientific, catalog number: AXYRESV25S)
2. Plate-sealing film (EXCEL Scientific, Inc., catalog number: EZP-100)
3. 96-well assay microplates (Corning, catalog number: 9017)
4. Polypropylene 96-well plate (Elkay, catalog number: MICR-PVX)
5. PYREX® 50 mL round media storage bottles (Corning, catalog number: 1395-50)
6. PYREX® 100 mL round media storage bottles (Corning, catalog number: 1395-100)
7. Universal pipette tips, 200 μL (VWR, catalog number: 76322-516)
8. Universal pipette tips, 300 μL (VWR, catalog number: 76322-520)
9. Universal pipette tips, 1,250 μL (VWR, catalog number: 76322-138)
10. 50 mL polypropylene conical tubes (Geiner Bio-One, catalog number: 227 261)
Equipment
1. Centrifuge (Beckman Coulter Microfuge 20, catalog number: B31600)
2. Microplate reader (Molecular Devices, model: SpectraMax® 190)
3. Vortex mixer (Vortex-Genie 2, model: G-560)
4. P20 Pipetman (Gilson, catalog number: F123600)
5. P200 Pipetman (Gilson, catalog number: F123601)
6. P1000 Pipetman (Gilson, catalog number: F123602)
7. 5–100 μL 8-channel electronic pipette (Eppendorf, Xplorer®, catalog number: 4861000120)
8. 15–300 μL 8-channel electronic pipette (Eppendorf, Xplorer®, catalog number: 4861000147)
9. 20–1,200 μL 8-channel electronic pipette (Eppendorf, Xplorer®, catalog number: 4861000163)
Software and datasets
1. SoftMax® Pro 4.8, by Molecular Devices
2. Microsoft Excel
Procedure
文章信息
稿件历史记录
提交日期: Oct 28, 2024
接收日期: Dec 16, 2024
在线发布日期: Jan 8, 2025
出版日期: Mar 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Donaghy, A. P., Schut, G. J., Shao, N., Poole, F. L. and Adams, M. W. W. (2025). Colorimetric Determination of Tungsten and Molybdenum in Biological Samples. Bio-protocol 15(5): e5195. DOI: 10.21769/BioProtoc.5195.
分类
生物化学 > 其它化合物 > 元素
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












