- EN - English
- CN - 中文
A PDMS-based Microfluidic Chip Assembly for Time-Resolved Cryo-EM (TRCEM) Sample Preparation
基于PDMS的微流控芯片组装用于时间分辨冷冻电镜(TRCEM)样品制备
发布: 2025年02月20日第15卷第4期 DOI: 10.21769/BioProtoc.5193 浏览次数: 3270
评审: Oneil Girish BhalalaNeha NandwaniMunenori Ishibashi
Abstract
Time-resolved cryo-EM (TRCEM) makes it possible to provide structural and kinetic information on a reaction of biomolecules before the equilibrium is reached. Several TRCEM methods have been developed in the past to obtain key insights into the mechanism of action of molecules and molecular machines on the time scale of tens to hundreds of milliseconds, which is unattainable by the normal blotting method. Here, we present our TRCEM setup utilizing a polydimethylsiloxane (PDMS)-based microfluidics chip assembly, comprising three components: a PDMS-based, internally SiO2-coated micromixer, a glass-capillary microreactor, and a PDMS-based microsprayer for depositing the reaction product onto the EM grid. As we have demonstrated in recent experiments, this setup is capable of addressing problems of severe sample adsorption and ineffective mixing of fluids and leads to highly reproducible results in applications to the study of translation. As an example, we used our TRCEM sample preparation method to investigate the molecular mechanism of ribosome recycling mediated by High frequency of lysogenization X (HflX), which demonstrated the efficacy of the TRCEM device and its capability to yield biologically significant, reproducible information. This protocol has the promise to provide structural and kinetic information on pre-equilibrium intermediates in the 10–1,000 ms time range in applications to many other biological systems.
Key features
• Design and fabrication of high-performance splitting-and-recombination-based micromixer and planar microsprayer.
• Protocol for SiO2 coating on the PDMS surface and fabrication of the microfluidic chip assembly.
• Preparation of time-resolved cryo-EM sample in the time range of 10–1,000 ms.
• Data collection on EM grid covered with droplets from the microsprayer.
Keywords: Microfluidics (微流控)Graphical overview
Background
Time-resolved cryo-electron microscopy (TRCEM) [1] is on the way to become a key technique to unlock the dynamics of biomolecular reactions by providing occupancies (i.e., kinetic data) and structural manifestations of intermediate states in the approximate time range of 10–1,000 ms. The setup of a TRCEM experiment must ensure thorough mixing of the reactants, precise control of reaction time, and fast vitrification, such that reaction intermediates can be trapped and subsequently visualized by single-particle cryo-EM.
Over the past two decades, many TRCEM methods have been developed [2–10]. These can be grouped into two main categories: spraying/mixing and mixing/spraying. In the first category, a sprayer or dispenser device deposits a reactant onto an EM grid that is pre-covered with another reactant. Both mixing and reacting occur on the EM grid, which is then rapidly plunged into the cryogen for fast vitrification. In this case, the length of the reaction time is controlled solely by the duration of the plunging. However, the uniformity of the reaction is not ensured as it relies on the efficiency of diffusive mixing on the grid and is affected by uncontrolled processes at the air–water interface. In the second category, a microfluidic chip combining the functions of mixing, reacting, and spraying is used for controlling the reaction and the deposition of the reaction product onto the grid. Here, the plunging time is kept very short to keep the on-grid time to a minimum. Most importantly, mixing and reacting can be separately manipulated, and the reaction time is defined mainly by the residence time of the sample in the microfluidic chip.
For the reasons stated, we have opted for the mixing/spraying microfluidics-based sample preparation method in our development of TRCEM technology. This development was driven by the need to migrate from silicon, in the initial version of the microfluidic chip [8], to plastic polymers as they are more versatile and cost-effective [4,5,10]. However, this migration faced an important obstacle since polymers such as IP-S and IP-Q photoresins and PDMS are intrinsically hydrophobic and prone to the adsorption of biomolecular samples, thereby impairing control over the reactions. Another problem we needed to address—this one shared with silicon-based chips—was ineffective mixing of fluids due to limited micromixer performance in the laminar flow regime.
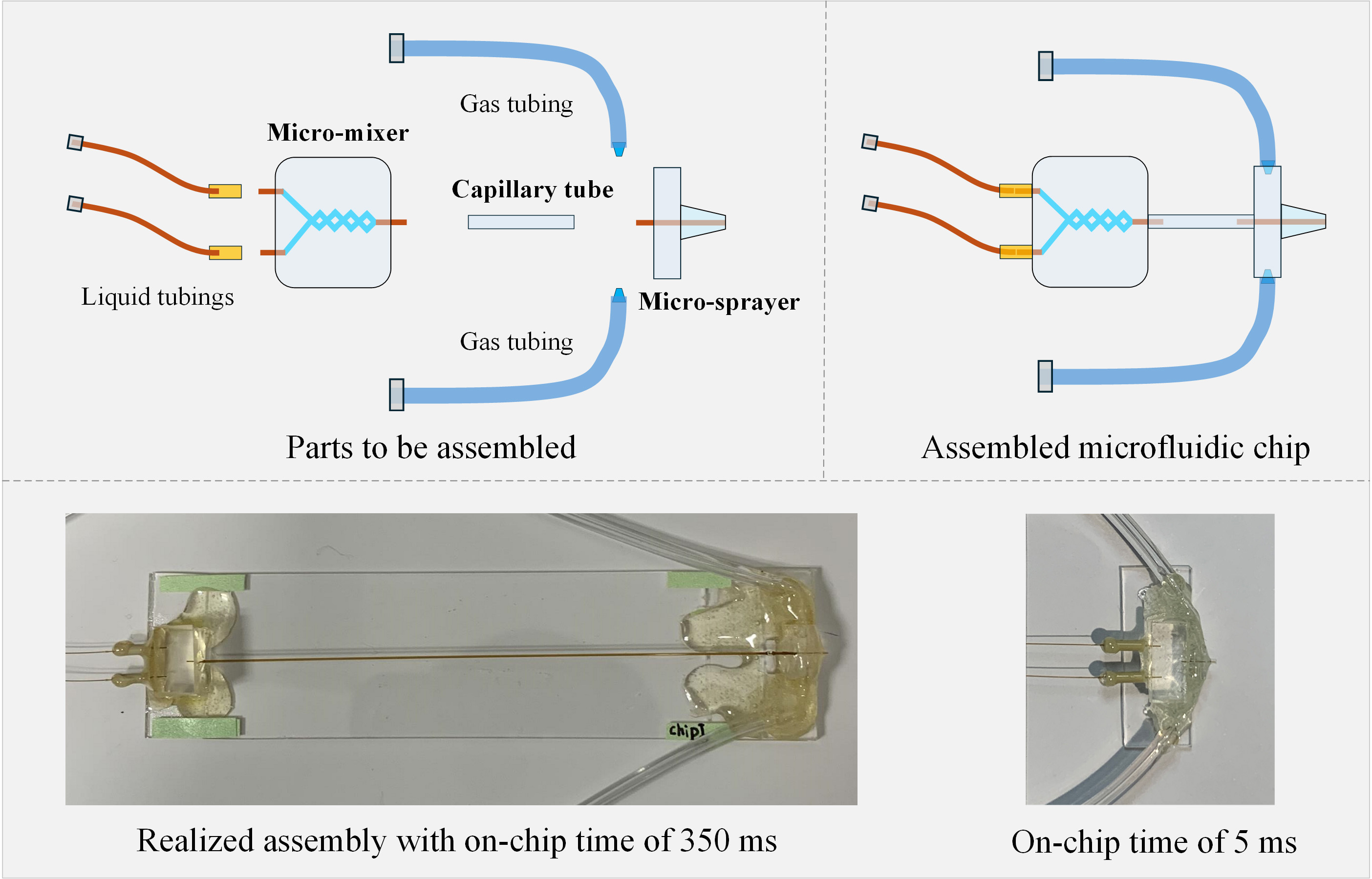
In our attempt to overcome these problems, we upgraded our TRCEM setup with a PDMS-based microfluidic chip assembly comprising three modules [11]: 1) a PDMS-based splitting-and-recombination-based (SAR) micromixer with 3D self-crossing channels, which is able to efficiently mix the solutions for uniform initiation of a reaction; 2) a polyimide-coated glass capillary tubing, which ensures precise flow rate control of liquids, to serve as the microreactor whose dimensions define the reaction time; 3) a PDMS-based microsprayer (with a design improved from our previous microsprayer [12]) employed to spray out the droplets of the reaction product onto the EM grid. This sprayer has been demonstrated in our work to be efficient for preparing cryo-EM grids with vitreous ice of controllable, highly consistent thickness. The details of the fabrication of these three modules, their integration into a functioning assembly mounted in the TRCEM apparatus, and the testing of the entire setup with biological samples are described here.
We believe that the protocol contains sufficient detail to allow other teams with appropriate scientific and engineering skills to fabricate the microfluidic chips, assemble the entire functioning apparatus, and duplicate the experiments described.
Materials and reagents
Biological materials
Note: Since this protocol is focused on the preparation of TRCEM grids, the purification of biological materials (E. coli 70S ribosome and HflX) will not be explained here. Note that HflX is known as a universally conserved protein for prokaryotic cells, a GTPase that functions as a rescue factor that splits the 70S ribosome into its 30S and 50S subunits in response to heat shock or exposure to antibiotics. For details, the reader is referred to [11] and literature cited therein.
1. E. coli 70S ribosome (1 mM in buffer solution)
2. HflX (5 mM in buffer solution)
Reagents
1. GTP (Invitrogen, catalog number: 18332015)
2. Methylene blue (Sigma-Aldrich, catalog number: 457250)
3. Tris base (Fisher Scientific, catalog number: BP152-1)
4. Ammonium chloride (NH4Cl) (Sigma-Aldrich, catalog number: 09718)
5. Magnesium acetate [Mg(OAc)2] (Sigma-Aldrich, catalog number: M5661)
6. 2-Mercaptoethanol (βME) (Sigma-Aldrich, catalog number: M6250)
Solutions
1. Methylene blue solution (see Recipes)
2. Buffer solution (see Recipes)
Recipes
1. Methylene blue solution (10 mL)
Use within 3 years and store at room temperature.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Methylene blue | 4% (w/v) | 2.5 mL |
| DI water | n/a | 7.5 mL |
| Total | 1% (w/v) | 10 mL |
2. Buffer solution (10 mL)
Use within one week and store at 4 °C.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris-HCl (1 M, pH 8.0) | 20 mM | 200 μL |
| NH4Cl (5 M) | 100 mM | 200 μL |
| Mg(OAc)2 (2 M) | 10 mM | 50 μL |
| βME (14.3 M) | 4 mM | 2.8 μL |
| DI water | n/a | 9.55 mL |
| Total | n/a | 10 mL |
Note: Add a predetermined amount of Tris base powder to DI water and adjust it to the desired pH value using HCl to obtain the Tris-HCl buffer (1 M, pH 8.0).
Laboratory supplies
1. Silicon wafer with 100 mm diameter (UniversityWafer, Inc. Category: Silicon, ID: 452)
2. Tweezers (Electron Microscopy Sciences, item number: 0508-L5-PO, https://www.dumonttweezers.com/Tweezer/TweezerStyle/42)
3. AB glue (Loctite, epoxy, dries clear, SKU: 1943587, https://www.loctiteproducts.com/products/central-pdp.html/loctite-clear-epoxy/SAP_0201OIL029V3.html)
4. Single-sided tape (Scotch Magic Tape, 0.75 in. × 650 in., https://www.amazon.com/Scotch-Dispensers-Applications-Invisible-Engineered/dp/B0000DH8HQ?ref_=ast_sto_dp)
5. Double-sided tape (Scotch Double Sided Tape, 0.75 in. × 300 in., https://www.amazon.com/Scotch-Double-Dispenser-Standard-237/dp/B0000DH8IT)
6. Aluminum foil (Reynolds, heavy duty, https://www.reynoldsbrands.com/products/aluminum-foil/heavy-duty-foil)
7. Scissors, surgical scissors, sharp blunt (Fine Science Tools, catalog number: 14001-12, https://www.finescience.com/en-US/Products/Scissors/Standard-Scissors/Surgical-Scissors-Sharp-Blunt/14001-12)
8. PDMS hole punchers (Ted Pella, Inc, Harris Uni-Core, Hole 1.0 mm and Hole 0.5 mm)
9. Cutting Mat (ALVIN Drafting, catalog number: HM1218, https://alvindrafting.com/products/hobby-mat-blue-gray)
10. Glass cutter tool set 2–20 mm pencil style oil feed carbide tip (MOARMOR, https://www.amazon.com/gp/product/B07Y1D243H/ref=ppx_yo_dt_b_search_asin_title?ie=UTF8&psc=1)
11. Sandpaper (S&F STEAD & FAST: https://www.amazon.com/STEAD-FAST-Sanding-Discs-Painting/dp/B0BKKNZRRW S&F STEAD & FAST 6 in. Sanding Discs Hook and Loop 24 Pcs, SKU: SA-SC-005)
12. EM grids (TED PELLA, INC., Quantifoil R 0.6/1 holey carbon copper grid, catalog number: 659-300-CU)
13. Tubings [Polymicro Technologies, TSP180350 with Outer diameter (O.D.) 360 μm and inner diameter (I.D.) 180 μm; TSP100170 with O.D. 170 μm and I.D. 100 μm; TSP075150 with O.D. 150 μm and I.D. 75 μm]
14. Pipette tips (BRANDTECH, 0.5–20 μL, colorless, catalog number: 732104, https://shop.brandtech.com/en/pipette-tips-0-5-20-l-pp-colorless.html; BIOTIX, 20 μL racked, sterilized, catalog number: 63300005 https://biotix.com/products/pipette-tips/xtip4-lts-compatible-pipette-tips/20-%CE%BCl-racked-sterilized-2/)
15. Pipette (Eppendorf, 0.1–5 μL)
16. Ethane gas (Airgas, grade level: research purity)
17. Liquid nitrogen (Airgas, gas grade: Industrial)
18. Vitrification dewar (Nanosoft, SKU: 21021005, https://www.nanosoftmaterials.com/product-page/vitrobot-dewar)
19. Tubes (Thermo Scientific, 15 mL Conical Sterile Polypropylene Centrifuge Tubes, model: 339651, https://www.thermofisher.com/order/catalog/product/339651)
20. Blades (https://uat.garveyproducts.com/Product/Index/7097, model: CUT-40475)
21. Photo mask (Fineline Imaging, Inc. https://www.fineline-imaging.com/)
22. 500 g weight (AMERICAN WEIGH SCALES, model: B00SSK3YNO)
23. Polyimide-coated, fused silica capillary tubing (Polymicro Technologies, TSP075150 with O.D. 150 μm and I.D. 75 μm)
24. SU-8 2050 (KAYAKU ADVANCED MATERIALS INC. SU-8 2050 500ML glass bottle with a cap, catalog number: NC0060520, https://www.fishersci.com/shop/products/NC0060520/NC0060520)
25. SU-8 developer (KAYAKU ADVANCED MATERIALS INC. Photoresist developer solution, catalog number: NC9901158, https://www.fishersci.com/shop/products/su-8-developer-4l/NC9901158)
26. Polydimethylsiloxane (PDMS) (Dow Corning, Sylgard 184, catalog number: 4019862)
27. Acetone (Pharmco-Aaper, Midland Scientific, catalog number: 329000000CSGF)
28. Isopropanol (Fisher Chemical, catalog number: BP26184)
29. Acid piranha [a 3:1 mixture of concentrated sulfuric acid (H2SO4) with hydrogen peroxide (H2O2)]; special protection equipment is required for preparing acid piranha [13], and formal training is highly recommended and required for the sake of safety
30. Nitrogen spray guns with 0.80 filter (Cleanroom World, catalog number: TA-NITRO-4-FT, https://cleanroomworld.com/cleanroom-equipment/nitrogen-spray-guns/90-degree-angle-gun-with-hose-assembly-half-inch-tubing); nitrogen is house-generated in Columbia clean room, industrial grade level
31. Polyimide tape (MYJOR, Model number: B07RZYY2T1, https://www.amazon.com/MYJOR-Temperature-Protect-Printer-Professionals/dp/B07RZYY2T1?th=1)
32. PEEK tubing (INDEX HEALTH & SCIENCE, Yellow, 1/16” × 0.007” × 5 ft, Part no.: 1536, https://www.idex-hs.com/store/product-detail/peek_tubing_yellow_1_16_od_x_007_id_x_5ft/1536)
Equipment
1. Optical microscope 1 (Leica, model: LEITZ DM IL) with a digital camera (AmScope, model: MU 1803-HS)
2. Optical microscope 2 (Nikon, model: ECLIPSE ME600L)
3. Optical microscope 3 (Nikon, model: ECLIPSE LV100ND) with a digital camera (Nikon, model: DS-Ri2)
4. Syringe pump (Cole Palmer Instrument Co., catalog number: 78-0200C)
5. Vacuum system (ZENYTM 3.5CFM 1/4HP Pump: https://www.zeny.us/collections/air-vacuum-pump/, model: VP 125+; Stainless Steel SlickVacSeal Vacuum Chamber: slickvacseal.com, Brand: SlickVacSealTM)
6. Glow-discharge machine (Ted Pella Inc., model: 91000S PELCO easiGlowTM Glow Discharge system for Cryo-EM)
7. Plasma processing system for plasma-enhanced chemical vapor deposition (PECVD) (Oxford Instruments Nanotechnology Tools Limited, model: PlasmaPro® NGP80)
8. Time-resolved apparatus for liquid pumping and pneumatic plunging (developed by Dr. Howard White, Eastern Virginia Medical School). More details about the apparatus can be seen in Section E.
9. Hot plate 1 (VWR International, LLC., catalog number: 97042-634)
10. Hot plate 2 (Electronic Micro Systems Ltd., model: 1000 PRECISION HOT PLATE)
11. Hot plate 3 (Electronic Micro Systems Ltd., model: 1000-1 PRECISION HOT PLATE)
12. Spincoating station (ReynoldsTech Fabricators, Inc., programmable FS5.0 spincoating station)
13. Mask aligner (SÜSS MicroTec, model: SUSS MA6)
14. Plasma system (ANATECH USA https://anatechusa.com/, model: Anatech SCE110)
15. Ultrasonic cleaner (Branson Ultrasonics Corp., model: B1510R-DTH)
Software and datasets
1. Nikon NIS-Elements (version 5.21.03, together with Microscope 2, purchased by Columbia University clean room)
2. Fiji/ImageJ (version 2.1.0/1.53c or another version, free and open source)
3. L-Edit (Win32 8.30, license is required); L-Edit is a mask layout editor for Windows-based platforms. For more details about the software, please contact the SIEMENS company: https://eda.sw.sie mens.com/en-US/ic/ic-custom/ams/l-edit-ic/
4. Electron Microscopy Data Bank (EMDB) (https://www.ebi.ac.uk/emdb/) EMD-29681, EMD-29688, EMD-29687, and EMD-29689
Procedure
文章信息
稿件历史记录
提交日期: Jul 9, 2024
接收日期: Dec 10, 2024
在线发布日期: Jan 8, 2025
出版日期: Feb 20, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Feng, X. and Frank, J. (2025). A PDMS-based Microfluidic Chip Assembly for Time-Resolved Cryo-EM (TRCEM) Sample Preparation. Bio-protocol 15(4): e5193. DOI: 10.21769/BioProtoc.5193.
分类
生物物理学 > 电子冷冻断层扫描
生物物理学 > 生物工程
生物化学 > 蛋白质 > 结构
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link