- EN - English
- CN - 中文
Development and Validation of Chlamydia muridarum Mouse Models for Studying Genital Tract Infection Pathogenesis
建立并验证小鼠衣原体生殖道感染病理研究模型
发布: 2025年02月05日第15卷第3期 DOI: 10.21769/BioProtoc.5181 浏览次数: 1291
评审: Andrea GramaticaPooja MukherjeeNidhi MenonJohn P Phelan
Abstract
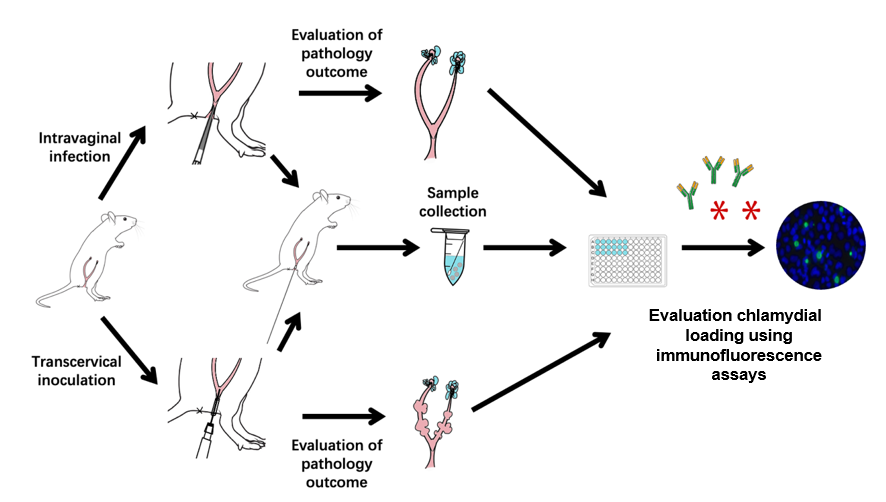
Animal infection models play significant roles in the study of bacterial pathogenic mechanisms and host–pathogen interactions, as well as in evaluating drug and vaccine efficacies. Chlamydia trachomatis is responsible for infections in various mucosal tissues, including the eyes and urogenital, respiratory, and gastrointestinal tracts. Chronic infections can result in severe consequences such as trachoma-induced blindness, ectopic pregnancy, and infertility. While intravaginal inoculation of C. muridarum mimics the natural route of sexual transmission between individuals, transcervical inoculation allows the organisms to directly infect endometrial epithelial cells without interference from host responses triggered by chlamydial contact or infection of vaginal and cervical cells. Therefore, in this study, we used mouse models to visualize pathologies in both the endometrium and oviduct following C. muridarum inoculation.
Key features
• This protocol develops the mouse-adapted Chlamydia muridarum model, ideal for visualizing pathologies in both the endometrium and oviduct genital tract.
• Requires female mice and utilizes specific techniques for intravaginal and transcervical inoculation with chlamydial elementary body (EB) and a form specialized for intracellular replication.
• The protocol necessitates specialized equipment, including a laminar flow hood, a micropipette, and a non-surgical embryo transfer device (NSET).
Keywords: Chlamydia muridarum (小鼠衣原体)Graphical overview
Background
All chlamydial species are obligate intracellular bacteria that replicate exclusively inside eukaryotic host cells. Chlamydial infections are dependent on a complex infection cycle that depends on transitions between specific forms. This cycle consists of a cell form specialized for host cell invasion, the elementary body (EB), and a form specialized for intracellular replication, the reticulate body (RB). Chlamydia trachomatis is a leading cause of sexually transmitted bacterial infections. Although C. trachomatis is sensitive to antibiotics, many infected individuals neglect medical treatment due to the lack of specific symptoms. The lack of treatment may allow chlamydial organisms to ascend to the upper genital tract, causing pelvic inflammatory diseases, ectopic pregnancy, or tubal infertility, depending on the upper genital tissues affected and the severity of the invasion by the chlamydial organisms. The current clinical challenges include no effective means for preventing chlamydial ascension and no approved C. trachomatis vaccines for preventing chlamydial infection or disease. Modeling chlamydial infection in the female mouse genital tract has provided a productive platform for revealing pathogenic and protective mechanisms during chlamydial infection [1].
The mouse-adapted C. muridarum can efficiently evade innate immunity in the lower genital tract and ascend to the upper genital tract where it induces long-lasting sequelae [2,3] such as hydrosalpinx, which is similar to that observed under laparoscopy in women infected with the human pathogen C. trachomatis. Thus, the C. muridarum model has been used to investigate the pathogenic mechanisms of long-term chlamydia infection [4–8]. In contrast, infection of the female mouse genital tract with C. trachomatis is limited and often fails to induce oviduct pathology [9]. Nevertheless, intravaginal inoculation with a high dose of C. trachomatis or transcervical inoculation with a moderate dose can cause glandular duct dilation, a pathology that was initially identified in C57 mice infected with C. muridarum [10]. Glandular duct dilation is observed as uterine horn dilation macroscopically. Since hydrosalpinx and uterine horn dilation can both lead to infertility, these pathologies have been used for evaluating chlamydial pathogenicity. Therefore, in this study, we used mouse models to study the pathology of the endometrium and oviduct following C. muridarum inoculation.
Materials and reagents
Biological materials
1. Mice: female (15–18 g), ≥ 6 weeks old, with strains and genotypes (wild type, transgenic, or knockout) to be determined depending on the specific experiment and model chosen. Mice were bred and maintained under specific pathogen-free conditions and on a standard chow diet for 8 weeks starting at 6 weeks old in the institutional animal facility of Shanghai Institute of Immunity and Infection
2. HeLa cells (human cervical epithelial carcinoma cells) (ATCC, catalog number: CCL2)
Reagents
1. Medroxyprogesterone acetate (Depo-Provera, Amphastar Pharmaceuticals, Inc, catalog number: 5401); resuspend in sterile 1× PBS (see Recipes) at a final concentration of 25 mg/mL
2. MD-76®R (NDC, catalog number: 0019-1317-09)
3. Trypan blue staining solution (Servicebio, catalog number: G1019-10ML)
4. 0.25% Trypsin-EDTA (1×), (Gibco, catalog number: 25200056)
5. 4% paraformaldehyde (Beyotime, catalog number: P0099-500 ml)
6. 0.1% Triton-X (Biosharp, catalog number: BL934B)
7. 3% BSA (COOLABER SCIENCE&TECHNOLOGY, catalog number: SL1331)
8. Disposable vacuum filters (500 mL, 0.22 μm) (BIOFIL, FMC201500)
9. DMEM (Gibco, catalog number: 11965092)
10. Heat-inactivated fetal bovine serum (FBS) (ExCell, catalog number: FND500)
11. Gentamicin, 10 mg/mL (BBI, catalog number: A428430)
12. Cycloheximide, 10 mg/mL (MCE, catalog number: HY-12320)
13. Sucrose (Sigma-Aldrich, catalog number: S9378)
14. Potassium phosphate, monobasic (Sigma-Aldrich, catalog number: P5655)
15. Potassium phosphate, dibasic trihydrate (Sigma-Aldrich, catalog number: P9666)
16. L-glutamic acid monosodium salt (Sigma-Aldrich, catalog number: G1626)
17. Sodium phosphate, dibasic (BBI, catalog number: A610879)
18. Sodium chloride (BBI, catalog number: A610476)
19. Potassium chloride (BBI, catalog number: A610440)
20. Potassium phosphate, monobasic (BBI, catalog number: A424391)
Solutions
1. Cycloheximide + gentamicin (C&G) growth medium (see Recipes)
2. MD-76R solutions, 52%, 44%, 40% (see Recipes)
3. Sucrose-phosphate-glutamate (SPG) buffer (see Recipes)
4. 1× PBS (see Recipes)
Recipes
1. Cycloheximide + gentamicin (C&G) growth medium
| Reagent | Volume |
|---|---|
| DMEM | 500 mL |
| Heat-inactivated fetal bovine serum | 50 mL |
| Gentamicin, 10 mg/mL | 1 mL |
| Cycloheximide, 10 mg/mL | 250 μL |
Store at 4 °C.
2. MD-76R solutions, 52%, 44%, 40%
| Solution | Reagents |
|---|---|
| 52% MD-76R | 52 mL MD-76R + 48 mL ddH2O |
| 44% MD-76R | 44 mL MD-76R + 56 mL ddH2O |
| 40% MD-76R | 40 mL MD-76R + 60 mL ddH2O |
Adjust to a total volume of 100 mL with sterile water and store at 4 °C.
3. Sucrose-phosphate-glutamate (SPG) buffer
| Reagent | Quantity |
|---|---|
| Sucrose | 74.62 g |
| Potassium phosphate, monobasic | 0.51 g |
| Potassium phosphate, dibasic trihydrate | 1.23 g |
| L-glutamic acid monosodium salt | 0.82 g |
| ddH2O | 1,000 mL |
| Total | 1,000 mL |
Mix with a stir bar until homogenous. Then, filter-sterilize the SPG buffer using disposable vacuum filters (500 mL, 0.22 μm, BIOFIL, FMC201500) and store at room temperature.
4. 1× PBS, pH 7.2
| Reagent | Quantity |
|---|---|
| Sodium phosphate, dibasic | 1.44 g |
| Sodium chloride | 8 g |
| Potassium chloride | 0.2 g |
| Potassium phosphate, monobasic | 0.24 g |
| ddH2O | 1,000 mL |
| Total | 1,000 mL |
Mix with a stir bar until homogenous. Sterilize PBS buffer by autoclaving and store at room temperature.
Laboratory supplies
1. 1 mL syringe & 27-gauge needle (Becton, Dickinson and Company, catalog number: 305109)
2. 1.5 mL microcentrifuge tubes (e.g., Fisher Scientific, catalog number: 21-402-903 or equivalent)
3. Speculum and non-surgical embryo transfer device (NSET) (ParaTechs, catalog number: 60010 or equivalent)
4. 96-well flat bottom cell culture plates, TC-treated (BIOFIL, catalog number: TCP010096)
5. Cell counting plate (Countstar, catalog number: CO010101)
Equipment
1. Microscope (YueHe, model: YHF40)
2. Centrifuge for 96-well plate (Eppendorf, model: Centrifuge 5810 R)
3. Laminar flow hood (Nuaire BSC Class II or equivalent)
4. Multi-channel pipettor (RAININ, model: L12-200XLS+)
5. P20 micropipette (Gilson, model: TB27868 or equivalent)
6. Fluorescence microscope (Olympus, model: AX70) with a CCD camera (Hamamatsu)
7. Ultracentrifuge (Beckman Coulter, model: Optima L-100K) with swinging bucket rotor (Beckman, model: SW 28 Ti or equivalent)
8. Superspeed centrifuge (Thermo Scientific, model: Sorvall RC 6 Plus) with fixed-angle rotor (Sorvall SS 34 or equivalent)
9. Sonics VCX 130 sonicator (Sonics & Materials, model: VCX 130 or equivalent).
10. Countstar Altair (Shanghai Ruiyu Biotechnology Co., Ltd)
Procedure
文章信息
稿件历史记录
提交日期: Aug 3, 2024
接收日期: Nov 28, 2024
在线发布日期: Dec 26, 2024
出版日期: Feb 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wang, Y., Han, Z., Wang, L., Sun, X., Tian, Q. and Zhang, T. (2025). Development and Validation of Chlamydia muridarum Mouse Models for Studying Genital Tract Infection Pathogenesis. Bio-protocol 15(3): e5181. DOI: 10.21769/BioProtoc.5181.
分类
微生物学 > 体内实验模型 > 原生动物
细胞生物学 > 模式生物培养
医学 > 发炎
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link