- EN - English
- CN - 中文
Quantitative, Dynamic Detection of Neuronal Na+ Transients Using Multi-photon Excitation and Fluorescence Lifetime Imaging (FLIM) in Acute Mouse Brain Slices
利用多光子激发和荧光寿命成像技术(FLIM)在急性小鼠脑切片中定量动态检测神经元Na+瞬变
发布: 2025年02月05日第15卷第3期 DOI: 10.21769/BioProtoc.5175 浏览次数: 1734
评审: Alberto RissoneRaniki KumariAnonymous reviewer(s)
Abstract
Fluorescence lifetime imaging microscopy (FLIM) is a highly valuable technique in the fluorescence microscopy toolbox because it is essentially independent of indicator concentrations. Conventional fluorescence microscopy analyzes changes in emission intensity. In contrast, FLIM assesses the fluorescence lifetime, which is defined as the time a fluorophore remains in an excited state before emitting a photon. This principle is advantageous in experiments where fluorophore concentrations are expected to change, e.g., due to changes in cell volume. FLIM, however, requires collecting a substantial number of photons to accurately fit distribution plots, which constrains its ability for dynamic imaging. This limitation has recently been overcome by rapidFLIM, which utilizes ultra-low dead-time photodetectors in conjunction with sophisticated rapid electronics. The resulting reduction in dead-time to the picosecond range greatly enhances the potential for achieving high spatio-temporal resolution. Here, we demonstrate the use of multi-photon-based rapidFLIM with the sodium indicator ION NaTRIUM Green-2 (ING-2) for the quantitative, dynamic determination of Na+ concentrations in neurons in acute rodent brain tissue slices. We describe the loading of the dye into neurons and present a procedure for its calibration in situ. We show that rapidFLIM not only allows the unbiased determination of baseline Na+ concentrations but also allows dynamic imaging of changes in intracellular Na+, e.g., induced by inhibition of cellular ATP production. Overall, rapidFLIM, with its greatly improved signal-to-noise ratio and higher spatio-temporal resolution, will also facilitate dynamic measurements using other FLIM probes, particularly those with a low quantum yield.
Key features
• RapidFLIM of the sodium indicator ING-2 enables the intensity-independent recording of neuronal Na+ transients at unparalleled full frame rates of 0.5–1 Hz.
• RapidFLIM is essentially independent of dye concentrations and therefore not affected by dye bleaching.
• Full in situ calibrations enable the quantification of intracellular Na+ changes at high spatio-temporal resolution.
• RapidFLIM of ING-2 allows unbiased determination of cellular Na+ loading also in conditions of strong cell swelling.
Keywords: ING-2 (ING-2)Graphical overview
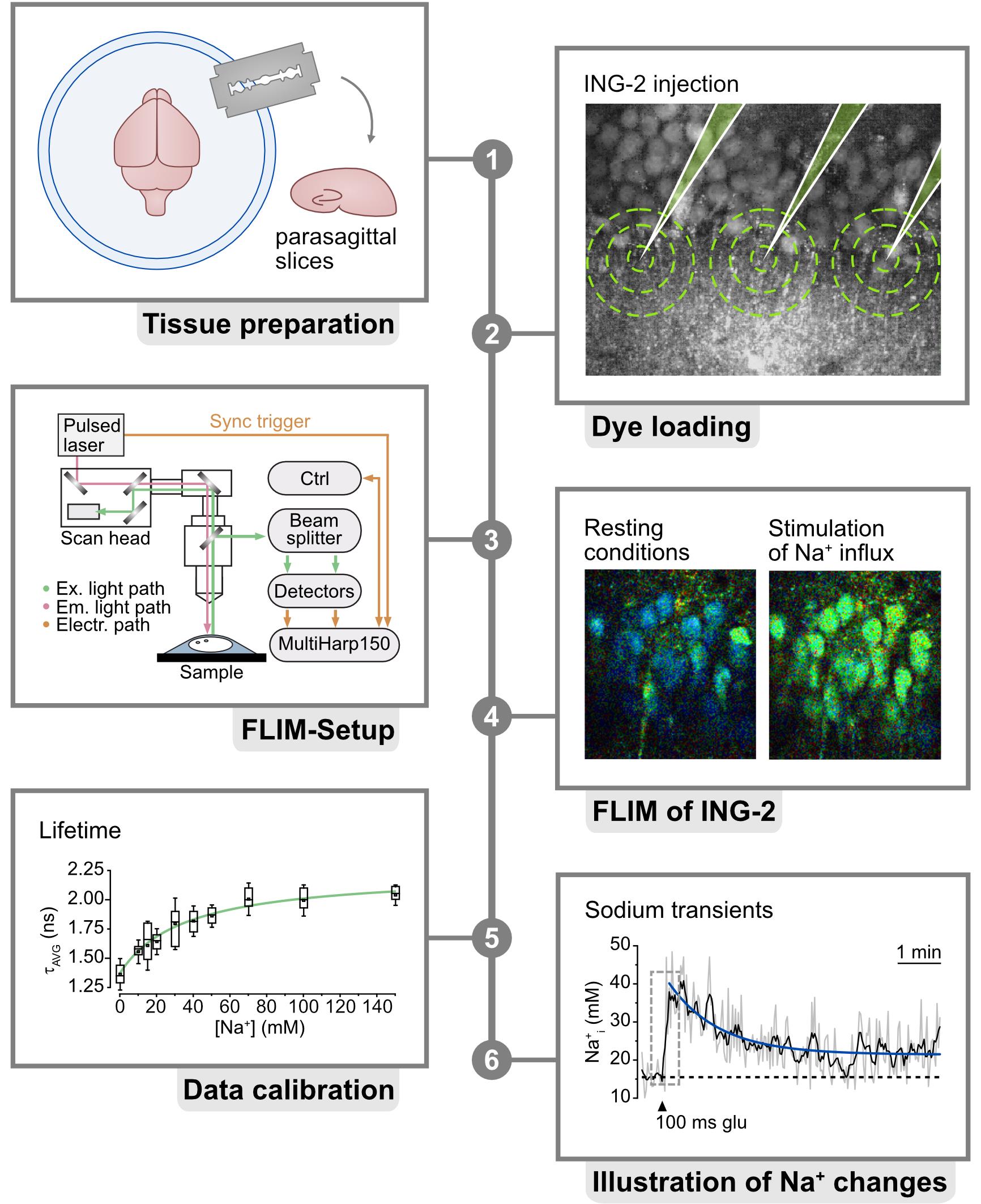
Background
Fluorescence lifetime imaging microscopy (FLIM) has emerged as an important technique for gaining a deeper understanding of ion concentration dynamics inside cells of the central nervous system [1–4]. In contrast to widely used intensity-based imaging, FLIM provides information based on the fluorescence lifetime, which is the duration that a fluorophore remains in an excited state before photon emission [5]. For many chemical indicator dyes, such as the widely used calcium indicator Oregon Green 488 BAPTA-1 [1,6], this excited state lifetime is correlated to ion binding. FLIM therefore enables a direct, quantitative readout of ion concentrations, provided that appropriate calibrations are carried out. Importantly, the fluorescence lifetime is largely independent of the concentration of the indicator dyes. This is particularly advantageous in experimental conditions where fluorophore concentrations change, e.g., due to bleaching or changes in cell volume [7]. However, traditional FLIM is subject to so-called dead-time artifacts, which greatly reduce the number of usable photons [8]. Dead-time artifacts result in the occurrence of seemingly shorter lifetimes when the photon count rates exceed a specific threshold, typically between 1% and 5% of the laser repetition rate. This necessitates the use of long collection times, of up to over one minute, to adequately fit the decay of the photon arrival times [9]. The resulting decrease in temporal resolution restricts the ability of FLIM for fast dynamic imaging.
The development of rapidFLIM, which reduces the dead time from approximately 100 ns to roughly 0.7 ns, has overcome this technical issue, allowing much faster repetition rates [10,11]. This enables the FLIM-based dynamic recording of transient changes in ion concentrations that are characteristic of cells of the nervous system, such as fluctuations in the intracellular Na+ concentration [3]. Na+ is integral to brain function, exerting a profound influence on both neurons and astrocytes through a multitude of cellular mechanisms. Disruptions in Na homeostasis result in altered neuronal excitability and impaired neurotransmission and contribute to the development of neurological disorders [12,13]. FLIM measurements of the intracellular Na+ concentration can be performed using the chemical indicator dye ION NaTRIUM Green-2 (ING-2), which shows an approximately 1–1.5-fold change in fluorescence lifetime upon Na+ binding [3,14,15]. ING-2 offers a relatively broad two-photon cross-section, allowing excitation within the range of 750–1,000 nm, while emission peaks between 500 and 600 nm [14,15]. Recently, we demonstrated for the first time that multi-photon-based, rapidFLIM enables dynamic imaging of Na+ transients in neurons in acute hippocampal tissue slices at so far unprecedented spatio-temporal resolution. Notably, this also allowed unbiased quantitative measurement of changes in neuronal Na+ induced by chemical ischemia, i.e., in conditions of strong cell swelling [3]. The present protocol describes the procedures for rapidFLIM for the quantitative recording of intracellular Na+ with ING-2, including the procedures for in situ calibration.
Materials and reagents
Biological materials
1. Balb/c mice bred by the Animal Care and Use Facility of the Heinrich Heine University Düsseldorf. Alternatively, other suitable rodent models can be used (e.g., Janvier, BALB/cJRj)
Reagents
1. Sodium chloride (NaCl) (Roth, catalog number: 3957)
2. Potassium chloride (KCl) (Roth, catalog number: 6781.1)
3. Calcium chloride dihydrate (CaCl2) (Fluka/Honeywell, catalog number: 31307)
4. Magnesium chloride hexahydrate (MgCl2) (Roth, catalog number: 2189.1)
5. Di-sodium hydrogen phosphate (Na2HPO4) (Roth, catalog number: 4984.1)
6. Sodium hydrogen carbonate (NaHCO3) (Applichem, catalog number: 131965)
7. Glucose (Caelo, catalog number: 2580)
8. 2-[4-(2-Hydroxyethyl)-1-piperazine]ethanesulfonic acid (HEPES) (Fisher, catalog number: BP310-500)
9. Magnesium sulfate (MgSO4) (Sigma-Aldrich, catalog number: 230391)
10. D-Gluconic acid sodium salt (Na-gluconate) (Sigma-Aldrich, catalog number: G9005-500G)
11. Gluconic acid potassium salt (K-gluconate) (Roth, catalog number: 2621)
12. Monensin sodium salt (Alfa Aesar, catalog number: J61669)
13. Potassium iodide (Thermo Fisher, catalog number: A12704.18)
14. Erythrosin B analytical standard, ≥ 98.0% (HPLC) (Erythrosin B) (Sigma-Aldrich, catalog number: 87613)
15. Gramicidin (Sigma-Aldrich, catalog number: G5002)
16. Ouabain octahydrate (Merck, catalog number: 4995)
17. ION NaTRIUM GreenTM-2 acetoxymethyl ester (ING-2 AM) (Mobitec, catalog number: 2011F)
18. PluronicTM F-127 (Pluronic) (Invitrogen, catalog number: P6867)
19. Sulforhodamine 101 (SR101) (Sigma-Aldrich, catalog number: S7635-50MG)
20. Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D8418)
Solutions
1. Artificial cerebrospinal fluid (standard ACSF) (see Recipes)
2. Modified ACSF for preparation of brain slices (preparation ACSF) (see Recipes)
3. HEPES-based ACSF (see Recipes)
4. ACSF for calibration (see Recipes)
5. SR101 stock solution (see Recipes)
6. ING-2 AM stock solution (see Recipes)
7. Bolus-loading solution (see Recipes)
8. Instrument response function solution (IRF solution) (see Recipes)
Recipes
1. Standard ACSF
| Reagent | Final concentration in double-distilled water |
|---|---|
| NaCl | 130 mM |
| KCl | 2.5 mM |
| CaCl2 | 2 mM |
| MgCl2 | 1 mM |
| NaH2PO4 | 1.25 mM |
| NaHCO3 | 26 mM |
| Glucose | 10 mM |
Adjust pH to 7.4 by bubbling with carbogen (95% O2 and 5% CO2).
The recommended overall volume is 1,000 mL.
2. Preparation ACSF
| Reagent | Final concentration in double-distilled water |
|---|---|
| NaCl | 130 mM |
| KCl | 2.5 mM |
| CaCl2 | 0.5 mM |
| MgCl2 | 6 mM |
| NaH2PO4 | 1.25 mM |
| NaHCO3 | 26 mM |
| Glucose | 10 mM |
Adjust pH to 7.4 by bubbling with carbogen (95% O2 and 5% CO2).
The recommended overall volume is 1,000 mL.
3. HEPES-based ACSF
| Reagent | Final concentration in double-distilled water |
|---|---|
| NaCl | 125 mM |
| KCl | 3 mM |
| CaCl2 | 2 mM |
| MgSO4 | 2 mM |
| NaH2PO4 | 1.25 mM |
| HEPES | 25 mM |
| Glucose | 10 mM |
Adjust pH to 7.4 by titration with NaOH.
The recommended overall volume is 100 mL.
4. ACSF for calibration
Note: Prepare calibration solutions with different sodium concentrations (e.g., 0, 10, 50, and 100 mM) by adjusting the concentration of K+, maintaining osmolarity. Solutions need to be prepared freshly every day.
| Reagent | Final concentration in double-distilled water |
|---|---|
| Na+ + K+ | 150 mM |
| Cl- | 30 mM |
| Gluconic acid | 120 mM |
| HEPES | 10 mM |
| MgSO4 | 2 mM |
| CaCl2 | 2 mM |
| Gramicidin | 3 μM |
| Monensin | 10 μM |
| Ouabain | 1 mM |
Adjust pH to 7.4 by titration with KOH.
The recommended overall volume is 500 mL for each.
5. SR101 stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| SR101 | 1.5 mM | - |
| Double-distilled H2O | - | 1 mL |
6. ING-2 AM stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| ING-2 AM | 1 mM | - |
| Pluronic | 20 mg/mL | - |
| DMSO | - | 46.12 μL |
7. Bolus-loading solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| ING-2 AM stock solution | 125 μM | 2 μL |
| HEPES-based ACSF | - | 14 μL |
8. IRF Solution
Note: Dissolve potassium iodide in H2O to saturation. Then, add 0.5 mg of Erythrosin B per 5 mL of H2O.
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Potassium iodide | Until saturation | - |
| Double-distilled H2O | - | 5 mL |
| Erythrosin B | - | 0.5 mg |
Laboratory supplies
1. Cyanoacrylate glue (e.g., Superflex gel, UHU)
2. Razor blades (e.g., razor blades classic, Wilkinson sword)
3. Scalpel blades (e.g., Heinz Herenz)
4. Filter paper (e.g., plain disc filter paper, Lab Logistics Group GmbH)
Equipment
1. Microtome (e.g., Campden Instruments, model: Campden 7000smz-2)
2. Micropipette puller (e.g., Narishige, model: PC-100)
3. Micromanipulator (e.g., Luigs & Neumann, model: LN Junior)
4. Pressure application device for dye injection (e.g., NPI Electronic GmbH, model: PDES)
5. Standard harp slice grids (e.g., ALA Scientific, model: HSG-5A or Warner SHD 41/10)
6. Photon count rate-optimized and custom-modified multiphoton laser imaging system (Figure 1) (a) with a high-intensity pulsed laser source (b) and time-correlated single photon counting (TCSPC) unit for FLIM data acquisition (c), e.g.,
a. Nikon A1MP (Nikon Europe) (Figure 1, steps 1–3)
b. MaiTai DeepSee (Newport Spectra-Physics GmbH, MKS) (Figure 1, step 4)
c. Multiharp 160, PicoQuant GmbH (Figure 1, step 3–4)
Custom modifications mainly include hardware and optical adaptations in the microscope, the NDD stage, and the connection between the microscope and the FLIM detector unit to prevent the reduction of the emission signal intensity going to the FLIM detection unit. These kinds of modifications require the help of both mechanical and electronic workshops and the support of the microscope manufacturer.
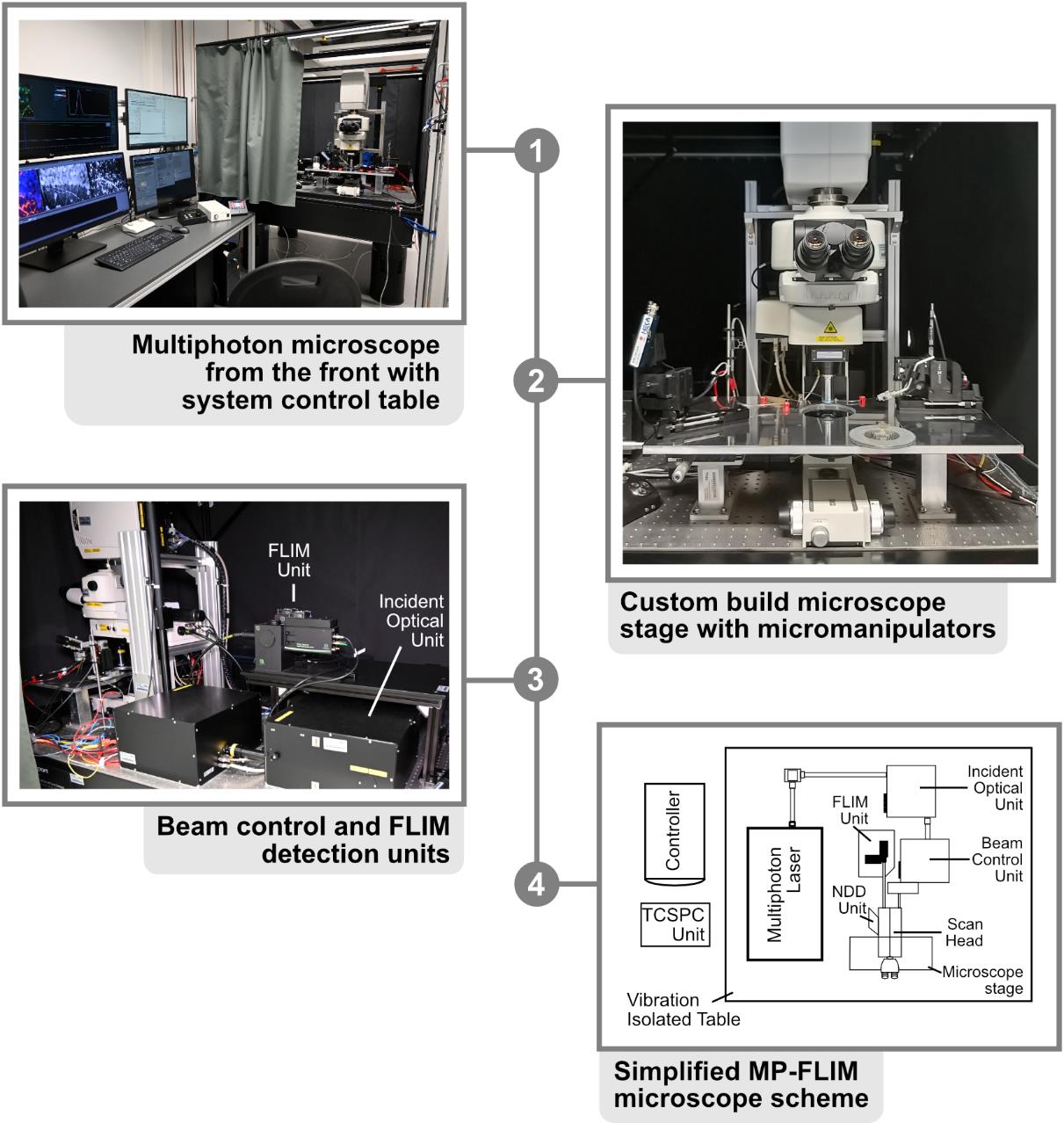
Figure 1. Multiphoton microscope setup. 1. Front view of the Nikon A1R MP with system control table. All software necessary for acquisition is started on the adjacent workstations and visible on the respective monitors. 2. Close-up of the microscope stage, with the slice chamber and micromanipulators present in the middle. 3. Backside of the Nikon A1R MP with customized emission port. FLIM unit and incident optical unit are visible in the middle. 4. Simplified scheme of the beam path. In the case of the A1R MP, the excitation beam is directed from the laser to the incident box and optical unit, where it is guided to the scan head of the microscope. After exciting the probe, the emission beam is directed to the FLIM detectors with as few protrusions (e.g., mirrors and filters) as possible, for maximal photon collection efficacy.
Software and datasets
1. NIS Elements Advanced Research (5.21, 08/28/2024, Nikon)
2. SymPhoTime 64 (2.8, 08/28/2024, PicoQuant)
3. OriginPro® (2021, 08/28/2024, OriginLab)
4. Microsoft Excel (Office 2021, 08/28/2024, Microsoft Corporation)
5. Affinity Designer [1.10, 08/28/2024, Serif (Europe) Ltd.]
Procedure
文章信息
稿件历史记录
提交日期: Sep 13, 2024
接收日期: Dec 3, 2024
在线发布日期: Dec 17, 2024
出版日期: Feb 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Eitelmann, S., Kafitz, K. W., Rose, C. R. and Meyer, J. (2025). Quantitative, Dynamic Detection of Neuronal Na+ Transients Using Multi-photon Excitation and Fluorescence Lifetime Imaging (FLIM) in Acute Mouse Brain Slices. Bio-protocol 15(3): e5175. DOI: 10.21769/BioProtoc.5175.
- Meyer, J., Gerkau, N. J., Kafitz, K. W., Patting, M., Jolmes, F., Henneberger, C. and Rose, C. R. (2022). Rapid Fluorescence Lifetime Imaging Reveals That TRPV4 Channels Promote Dysregulation of Neuronal Na+ in Ischemia. J Neurosci. 42(4): 552–566.
分类
神经科学 > 神经解剖学和神经环路 > 荧光成像
神经科学 > 神经系统疾病 > 细胞机制
细胞生物学 > 细胞成像 > 活细胞成像
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link