- EN - English
- CN - 中文
Rapid Sampling of Large Quantities of Interstitial Fluid from Human Skin Using Microneedles and a Vacuum-assisted Skin Patch
利用微针和真空辅助皮肤贴片快速采集大量人体皮肤间质液
发布: 2025年02月05日第15卷第3期 DOI: 10.21769/BioProtoc.5173 浏览次数: 2005
评审: Xiyuan LiuPilar Villacampa AlcubierreJessica Lauren Davis
Abstract
Interstitial fluid (ISF) is a promising diagnostic sample due to its extensive biomolecular content while being safer and less invasive to collect than blood. However, existing ISF sampling methods are time-consuming, require specialized equipment, and yield small amounts of fluid (<5 μL). We have recently reported a simple and minimally invasive technique for rapidly sampling larger quantities of dermal ISF using a microneedle (MN) array to generate micropores in the skin from which ISF is extracted using a vacuum-assisted skin patch. Here, we present step-by-step protocols for fabricating the MN array and skin patch, as well as for using them to sample ISF from human skin. Using this technique, an average of 20.8 μL of dermal ISF can be collected within 25 min, which is a ∼6-fold improvement over existing ISF sampling methods. Furthermore, the technique is well-tolerated and does not require the use of expensive or specialized equipment. The ability to collect ample volumes of ISF in a quick and minimally invasive manner will facilitate the analysis of ISF for biomarker discovery and its use for diagnostic testing.
Key features
• Minimally invasive (bloodless and nearly painless) technique for sampling ISF from human skin.
• An average of 20.8 μL of interstitial fluid can be collected within 25 min.
• This technique does not require expensive or specialized equipment or electricity.
• Collected ISF can be analyzed using conventional laboratory-based assays or point-of-care diagnostic tests.
Keywords: Microneedle (微针)Graphical overview
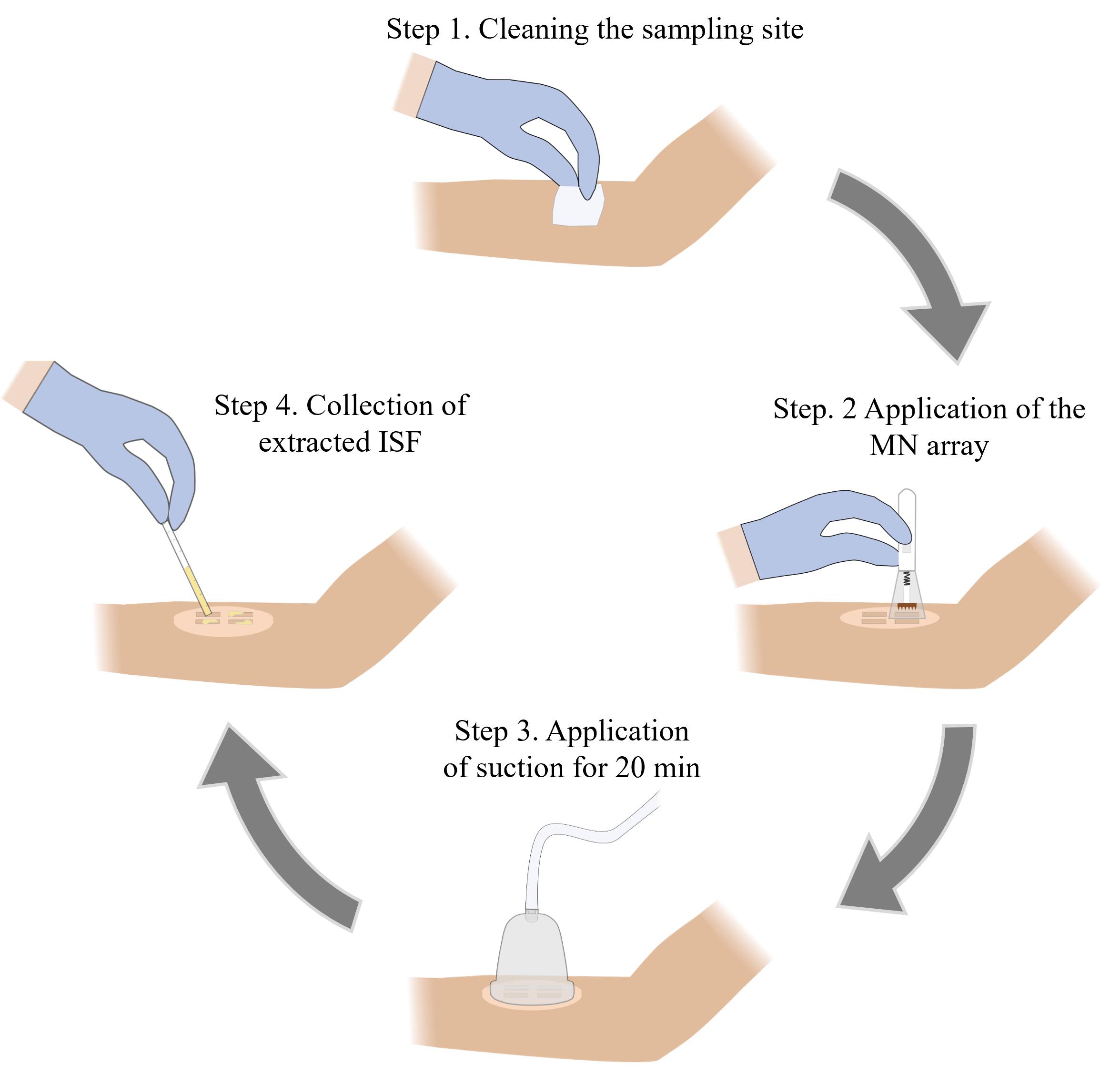
Microneedle (MN)-based sampling of dermal interstitial fluid (ISF) using a vacuum-assisted skin patch
Background
The diagnosis, prognosis, and monitoring of many diseases rely on the detection and/or quantification of biomarkers in blood. While blood sampling is a routine medical procedure, it poses risks of infection and can lead to complications, particularly in newborns and individuals with blood clotting disorders [1,2]. In addition, the discomfort associated with blood sampling can deter individuals with blood or needle phobias from getting tested [3,4]. Interstitial fluid (ISF), which is found in the extracellular space in tissues, has garnered much attention as a diagnostic fluid due to its similar biomarker content to blood [5–9]. Prior studies have shown that ISF collected from skin (dermal ISF) is a promising source for biomarkers (e.g., metabolites, proteins, nucleic acids, and exosomes). However, progress in the use of ISF as a diagnostic fluid is hampered by the lack of rapid and minimally invasive methods for collecting ample quantities of fluid [10,11].
Various methods for extracting ISF from skin have been reported, including the creation of suction blisters [7,8,12], microdialysis [13], open-flow microperfusion [14], laser microporation [15], and reverse iontophoresis [16]. While effective, these methods are time-consuming (~1 h), require the use of specialized equipment, and/or involve invasive procedures that can cause skin erythema and dehydration [7]. Microneedle (MN)-based techniques have also been demonstrated for the collection of human ISF, being faster and less invasive [5,12,17,18]. However, the fluid volumes collected from these techniques are too low (~1–5 μL) for biomolecular analysis using conventional diagnostic assays. For example, lateral flow immunochromatographic assays (LFIAs) require at least ~15 μL of sample, western blot requires ~15–60 μL of sample, and enzyme-linked immunosorbent assay (ELISA) requires 50–100 μL of sample.
We have recently reported a simple and minimally invasive method for sampling larger quantities of ISF from human skin [19]. This approach involves the use of a high-density MN array to generate micropores in the skin followed by the attachment of a rigid skin patch and application of mild vacuum pressure using a portable hand pump. The design of the MN array and the parameters associated with the sample collection process (number of MN insertions and the duration of vacuum application) were optimized in our prior study, and this protocol is based on these optimized parameters. The sampling efficiency of this technique was evaluated by collecting dermal ISF from 28 human volunteers, which yielded an average collection volume of 20.8 μL, a ∼6-fold improvement over existing ISF sampling methods. This technique was well-tolerated and reported as being nearly pain-free by all the volunteers. We envision that this protocol will be useful for physicians, scientists, and bioengineers interested in analyzing ISF for biomarkers that can be used for various diagnostic applications, including disease diagnosis and prognosis, as well as for monitoring therapeutic response.
Materials and reagents
Reagents
1. IP-Q photoresin (Nanoscribe, catalog number: 37071000), store in a UV-protected and airtight container in a cool dry environment away from direct sunlight at 4–8 °C; use only under yellow light with λ > 500 nm
2. SU-8 developer (Kayaku Advanced Materials, catalog number: Y020100)
3. Sylgard 184 silicone elastomer kit (Dow, SKU: 4019862)
4. SU-8 2025 photoresist (Kayaku Advanced Materials, catalog number: Y111069-0500L1GL), store in a UV-protected and airtight container in a cool dry environment away from direct sunlight at 4–21 °C; use only under yellow light with λ > 500 nm
5. Dichloro-p-cyclophane (Parylene C dimer) (Specialty Coating Systems, CAS: 28804-46-8)
6. 2-Propanol (IPA) >99.5% ACS (VWR, catalog number: BDH1133-4LG)
7. Deionized water (17.6 MΩ·cm)
8. Liquid nitrogen
9. SU-8 developer (EMD Performance Materials, AZ® Kwik Strip)
Solutions
1. Polydimethylsiloxane (PDMS) (see Recipes)
2. 70% IPA (see Recipes)
Recipes
1. Polydimethylsiloxane (PDMS)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 184 silicone elastomer base | n/a | 40 g |
| 184 silicone elastomer curing agent | n/a | 4 g |
| Total | n/a | 44 g |
2. 70% isopropyl alcohol (IPA)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 2-Propanol | 100% | 70 mL |
| Deionized water | n/a | 30 mL |
| Total | n/a | 100 mL |
Laboratory supplies
1. 3 mm thick clear poly(methyl methacrylate) (PMMA) sheet (McMaster Carr, catalog number: 87225K23)
2. 1.5 mm thick clear PMMA sheet (McMaster Carr, catalog number: 8574K51)
3. Polystyrene Petri dish (VWR, catalog number: 89230-472)
4. Plastic mixing cup (Fisher Scientific, catalog number: S04202)
5. Disposable stirring spatula (Millipore Sigma, catalog number: BR759800-500EA)
6. Razor blade (Bates, model: RZ50)
7. Kimwipes (Fisher Scientific, catalog number: 06-666)
8. Microscope glass slide (Fisher Scientific, catalog number: 125444)
9. Micropipette tips [Fisher Scientific, catalog numbers: 02-707-430 (0–200 μL), 02-707-432 (2–20 μL), 02-707-404 (100–1000 μL)]
10. 50 mL conical centrifuge tubes (Fisher Scientific, catalog number: 339653)
11. Double-sided microfluidic tape (3M Company, model: 9972A)
12. Medical-grade pressured-sensitive adhesive tape (Adhesives Research, model: 90106NB)
13. Alcohol prep pad (Fisher Healthcare, catalog number: 22-363-750)
14. Capillary tube, 70 μL (Fisher Scientific, catalog number: 22-260943)
15. Capillary plunger (CoaguSense, catalog number: 03P52-54)
16. Adhesive remover wipes (Smith & Nephew, catalog number: 402300)
17. 0.5 mL protein low-bind microcentrifuge tubes (Eppendorf, catalog number: 4030-8434)
18. 42 mm diameter vacuum cup and hand pump (Hansol Medical Equipment, model: 2018-01-26-0775)
19. Microneedle spring-loaded applicator (Micropoint Technologies)
20. Tweezers (Fisher Scientific, catalog number: 12-000-127)
21. 250 mL glass beaker (Corning Pyrex, catalog number: 1003-250)
22. Nitrile gloves (VWR, catalog number: 76518-336)
Equipment
1. Photonic lithography system (Nanoscribe, model: Photonic Professional GT+)
2. Forced air oven (VWR, catalog number: 89511-410)
3. 6 × 50 mL angle rotor and laboratory centrifuge (VWR, catalog numbers: 76181-202, 76181-190)
4. MiniSpin plus mini centrifuge (Eppendorf, catalog number: 022620207)
5. 50 W, 365 nm UV lamp (SUNUV)
6. Parylene deposition system (Specialty Coating Systems, model: PDS 2010 Labcoater)
7. Digital microscope (Keyence Corporation, model: VHX-7000)
8. CO2 laser cutter (Universal Laser Systems, model: VLS3.75)
9. Dremel MultiPro rotary tool (Dremel, model: 395)
10. Liquid nitrogen dewar (U.S. Solid, model: USS-LNT00001)
11. Nalgene vacuum chamber (Thermo Scientific, catalog number: 5305-0609)
12. Laboport N 816 pump (Fisher Scientific, catalog number: 13-880-31)
13. Portable precision balance (Ohaus, model: SPX2201)
14. Digital timer (Fisher Scientific, catalog number: 06-664-252)
15. Micropipette (Eppendorf, model: 2231300004)
Software and datasets
1. NX Student Edition (Siemens Digital Industries Software, Version 1934, 2020)
2. DeScribe (Nanoscribe, Photonic Professional GT+, 2021)
3. AutoCAD (Autodesk, Student version 2021)
4. Prism version 9.5 (GraphPad Software)
Procedure
文章信息
稿件历史记录
提交日期: Sep 13, 2024
接收日期: Dec 2, 2024
在线发布日期: Dec 13, 2024
出版日期: Feb 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Wilkirson, E. C., Jiang, X. and Lillehoj, P. B. (2025). Rapid Sampling of Large Quantities of Interstitial Fluid from Human Skin Using Microneedles and a Vacuum-assisted Skin Patch. Bio-protocol 15(3): e5173. DOI: 10.21769/BioProtoc.5173.
分类
生物工程 > 生物医学工程
医学
生物物理学 > 生物工程 > 医用生物材料
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












