- EN - English
- CN - 中文
High-resolution Cryo-EM Structure Determination of a-Synuclein—A Prototypical Amyloid Fibril
原型淀粉样纤维α-突触核蛋白的高分辨率冷冻电镜结构解析
发布: 2025年02月05日第15卷第3期 DOI: 10.21769/BioProtoc.5171 浏览次数: 3801
评审: Neha NandwaniVamseedhar RayaproluSuresh Kumar
Abstract
The physiological role of a-synuclein (a-syn), an intrinsically disordered presynaptic neuronal protein, is believed to impact the release of neurotransmitters through interactions with the SNARE complex. However, under certain cellular conditions that are not well understood, a-syn will self-assemble into β-sheet-rich fibrils that accumulate and form insoluble neuronal inclusions. Studies of patient-derived brain tissues have concluded that these inclusions are associated with Parkinson’s disease, the second most common neurodegenerative disorder, and other synuclein-related diseases called synucleinopathies. In addition, repetitions of specific mutations to the SNCA gene, the gene that encodes a-syn, result in an increased disposition for synucleinopathies. The latest advances in cryo-EM structure determination and real-space helical reconstruction methods have resulted in over 60 in vitro structures of a-syn fibrils solved to date, with a handful of these reaching a resolution below 2.5 Å. Here, we provide a protocol for a-syn protein expression, purification, and fibrilization. We detail how sample quality is assessed by negative stain transmission electron microscopy (NS-TEM) analysis and followed by sample vitrification using the Vitrobot Mark IV vitrification robot. We provide a detailed step-by-step protocol for high-resolution cryo-EM structure determination of a-syn fibrils using RELION and a series of specialized helical reconstruction tools that can be run within RELION. Finally, we detail how ChimeraX, Coot, and Phenix are used to build and refine a molecular model into the high-resolution cryo-EM map. This workflow resulted in a 2.04 Å structure of a-syn fibrils with excellent resolution of residues 36–97 and an additional island of density for residues 15–22 that had not been previously reported. This workflow should serve as a starting point for individuals new to the neurodegeneration and structural biology fields. Together, this procedure lays the foundation for advanced structural studies of a-syn and other amyloid fibrils.
Key features
• In vitro fibril amplification method yielding twisting fibrils that span several micrometers in length and are suitable for cryo-EM structure determination.
• High-throughput cryo-EM data collection of neurodegenerative fibrils, such as alpha-synuclein.
• Use of RELION implementations of helical reconstruction algorithms to generate high-resolution 3D structures of a-synuclein fibrils.
• Brief demonstration of the use of ChimeraX, Coot, and Phenix for molecular model building and refinement.
Keywords: Cryo-EM (冷冻电镜)Graphical overview

Graphical overview of a-synuclein fibrilization and cryo-EM structure determination. a-syn protein expression and purification is followed by a fibrilization protocol yielding twisting filaments that span several micrometers in length and are validated by negative stain transmission electron microscopy (NS-TEM). The sample is then vitrified, followed by cryo-EM data collection. Real-space helical reconstruction is performed in RELION to generate an electron potential map that is used for model building.
Background
Amyloid formation within neurons has been well documented to cause neurodegeneration in patients leading to a variety of diseases including Alzheimer’s (AH), Parkinson’s disease (PD), Lewy Body disease (LB), and multiple system atrophy (MSA) [1–3]. The formation of amyloids is due to protein aggregation resulting in helical, filamentous assemblies with cross β-sheet quaternary structure (Figure 1) [4]. Amyloid filaments interact with different cellular components such as membranes, cytoskeletal factors, and other filaments to form inclusion bodies that disrupt cellular processes and ultimately lead to cell death [2]. These inclusion bodies are prominent in the postmortem brains of patients who have suffered from these neurodegenerative diseases, and early investigation of inclusion bodies revealed the presence of filamentous a-synuclein (a-syn) [1,2]. a-syn is a small (14.4 kDa) intrinsically disordered protein whose physiological role remains elusive. a-syn has the capability to bind to the SNARE complex and associate with vesicles at the neuronal axon terminus, providing evidence that it may have an impact on neurotransmitter release, vesicle docking, and vesicle trafficking [5–8]. However, upon misfolding, a-syn first forms oligomeric aggregates that eventually undergo fibrilization; these fibrils display the highly ordered cross β-sheets classically found in amyloids [9,10]. These, in turn, form the extended filaments that cause neuropathological changes in the brain and are specifically responsible for PD, LB, and MSA. Diseases caused by a-syn in this manner are called synucleinopathies [11].
The high-resolution structure presented here of filamentous wild-type a-syn is of a helical filament composed of two protofilaments; each turn (or rung) of the filament is comprised of two copies (one per protofilament) of a-syn facing nearly 180° from each other (Figure 1). Between the monomers that make up each protofilament, there is a hydrophobic interface composed of residues 50–57, similar to previously solved structures of filamentous a-syn [12–14]. This interface is stabilized by salt bridges and pseudo-screw symmetry, as previously reported [12,13]. For a-syn, there are seven different missense familial mutations commonly found in patients who have a higher disposition for synucleinopathies (A30P, E46K, H50Q, G51D, A53E, A53T, and A53V) [15–21]. Interestingly, six of these familial mutations lie within the core of the structure and may cause destabilization, resulting in a variety of different fibril morphologies. The presence of polymorphism has been demonstrated particularly well through the analysis of in vitro a-syn fibrils. Fibril twist, crossover distance, packing arrangement, number of protofilaments, interface, and tertiary structure, among others, can vary greatly under different micro- and macro-environments. Many different environmental factors such as pH, salt concentration, temperature, quiescence, and post-translational modifications have an impact on fibril morphology. This has led to the documentation of more than 60 in vitro structural polymorphs of a-syn in the PDB [22,23]. These structural differences in the in vitro filaments can have direct effects on nucleation rates, seeding propensities, and even cytotoxicity [23]. Unfortunately, the ties between these structurally distinct in vitro polymorphs to those found in sarkosyl-insoluble brain-derived structures remain elusive. However, evidence suggests that different polymorphs may influence pathologies [24–26]. This is demonstrated by the difference in a-syn folds of the filaments extracted from patients diagnosed with MSA vs. PD [27].
The formation of the filaments responsible for synucleinopathies is propagated in brain tissue by primary nucleation events in which a-syn monomer spontaneously undergoes structural changes resulting in nucleation. This nucleation site can then recruit additional a-syn monomers to bind, thus elongating the fibril [28,29]. However, there can also be secondary nucleation events in which preformed fibrils are introduced into the cellular environment as “seeds” [30]. These seeding events are significantly more potent at fibril formation and elongation. Remarkably, seeds from a particular polymorph have been shown to recruit wild-type a-syn, provide a structural template, and form filaments expressing the polymorph of the seed regardless of whether the endogenous protein recruited is pathogenic or not [31]. A consequence of this prion-like self-replication is that a-syn fibrils may move from cell to cell, spreading cytotoxic polymorphs.
The introduction of polymorphism has a multifactorial effect on clinical treatments of neurodegenerative diseases. Our understanding of the implications associated with each polymorph on disease progression, pathology, and patient outcomes is very limited. In addition, the differences in folding, packing, or twists of each polymorph introduce complexities in binding sites, affinities, and accessibility for a “one size fits all” drug for synucleinopathies. This is further complicated by evidence that not only are there disease-specific morphisms but each synucleinopathy can exhibit patient-to-patient heterogeneity [32]. Thus, to overcome these challenges, explore new therapeutic targets, understand specific polymorph effects on neuropathology, and develop therapies with patient-specific approaches, solving both patient-derived and in vitro amyloid polymorphs should be explored.
Here, we describe a helical reconstruction workflow that we use to solve the structure of in vitro assembled filamentous a-syn to a global resolution of 2.04 Å. We purify a-syn filaments from a reaction in which fibril seeding material is combined with monomeric a-syn. The fibril seeding material provides a template for fibril elongation via monomer addition over a 6-week incubation period at 37 °C with shaking at 250 rpm. The purified a-syn filaments are then imaged using negative stain transmission electron microscopy (NS-TEM) to evaluate sample integrity and fibril concentration on the grid. The sample is then applied to grids and plunge frozen, and the vitrified grids are used for cryo-EM data collection. We provide a detailed protocol utilizing RELION to reconstruct a high-resolution cryo-EM electron potential map that is then used for building an atomic model of the fibril (Figure 1B, 1C, 1E). The steps presented here may be applied to studies of various amyloid fibrils and accelerate cryo-EM structure determination in the fields of neurodegenerative research and medicine.
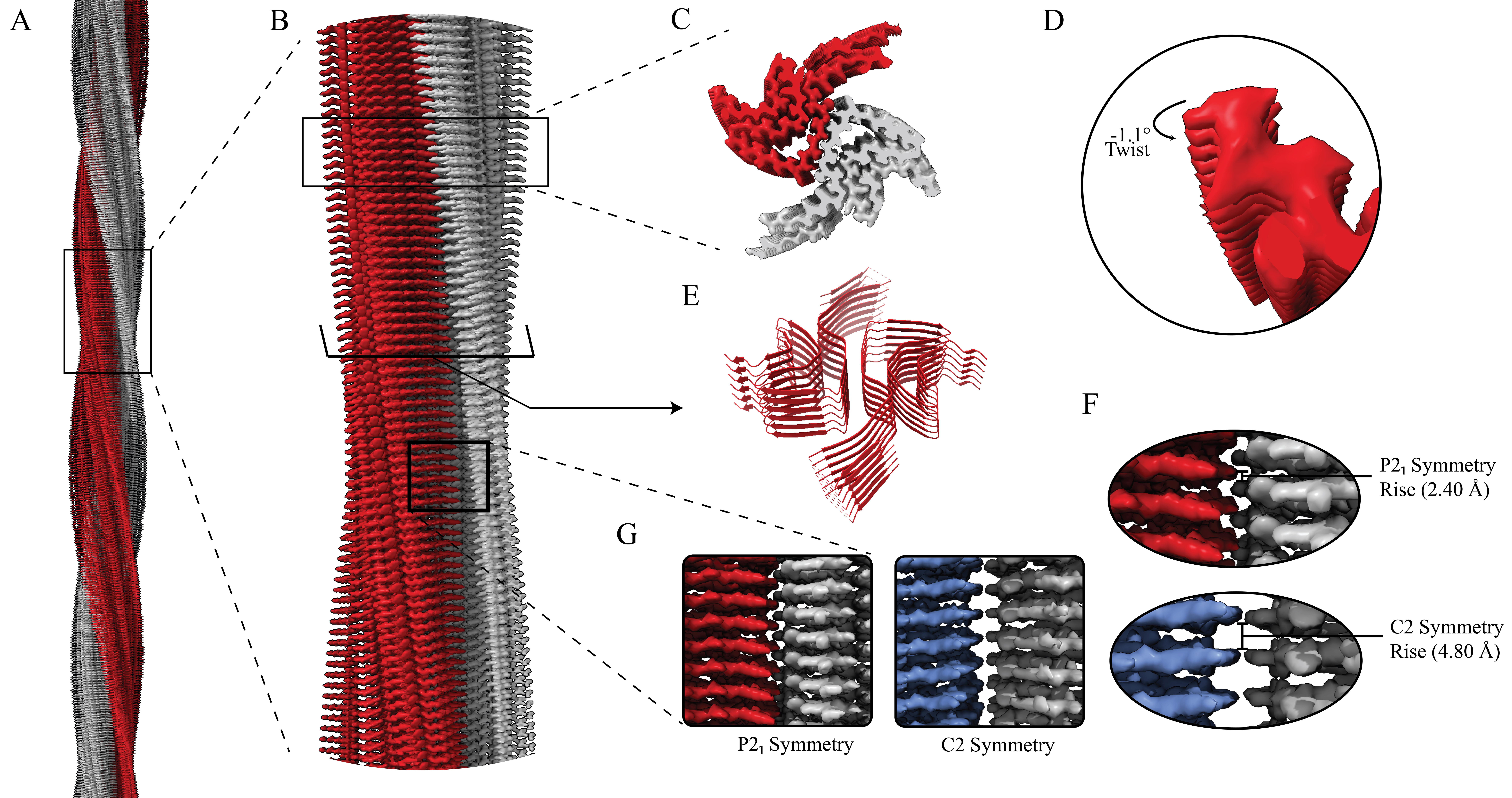
Figure 1. Structural features of a-syn fibrils from cryo-EM structures. A. Cryo-EM structure of full-length a-syn fibril depicting two protofilaments (one in red; one in grey). B. Magnified view of a-syn fibril portraying stacked rungs and filament twist. C. Cross-section of a-syn fibril electron potential map displaying two a-syn monomers that comprise each protofilament, approximately 180° from each other. D. Electron potential map of individual β-sheet stacks twisting. E. Model depicting the secondary structure of stacking β-sheets. F. Example of rise measurement for P21 symmetry (red) and C2 symmetry (blue). G. Possible packing symmetry between protofilaments for P21 symmetry (out of register) (red) and C2 symmetry (in register) (blue).
Materials and reagents
Biological materials
1. Plasmid with wild-type a-syn construct inE. coli BL21(DE3)/pET28a-AS [33]
Reagents
1. LB broth (Invitrogen, catalog number: 12780029)
2. Bacto agar (Dot Scientific Inc., catalog number: DSA20030-1000)
3. Magnesium sulfate (MgSO4) (Fisher Scientific, catalog number: 01-337-186)
4. Calcium chloride (CaCl2) (Fisher Scientific, catalog number: BP510-500)
5. Sodium phosphate (NaH2PO4) (Fisher Scientific, catalog number: 01-337-702)
6. Potassium phosphate (KH2PO4) (Fisher Scientific, catalog number: 01-337-803)
7. Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-500)
8. IPTG (Fisher Scientific, catalog number: BP1755-10)
9. Tris-HCl (Fisher Scientific, catalog number: PRH5125)
10. EDTA (Fisher Scientific, catalog number: AAA1516130)
11. Kanamycin monosulfate (Thermo Scientific, catalog number: J61272.14)
12. SDS-PAGE gels (Bio-Rad, catalog number: 4561096)
13. SDS-PAGE loading dye (Bio-Rad, catalog number: 1610737)
14. Coomassie Brilliant Blue (TCI, catalog number: 6104-59-2)
15. BME vitamins (Sigma-Aldrich, catalog number: B6891-100mL)
16. Sodium azide (Sigma-Aldrich, catalog number: 19-993-1)
17. Studier trace metal mix (Sigma-Aldrich, catalog number: 41106212)
18. Ammonium sulfate (Fisher Scientific, catalog number: A702-500)
19. Deuterium oxide (2H2O) (Cambridge Isotopes Laboratories, catalog number: DLM-4-1L)
20. BioExpress bacterial cell media 10× concentrate (U-13C, 98%; U-15N, 98%; U-D 98%) (Cambridge Isotopes Laboratories, catalog number: CGM-1000-CDN)
21.15N-NH4CI (Cambridge Isotopes Laboratories, catalog number: 39466-62-10)
22.2H-13C-glucose (Cambridge Isotopes Laboratories, catalog number: CDLM-3813-5)
23. Sodium deuteroxide (NaO2H) (Cambridge Isotopes Laboratories, catalog number: DLM-45-100)
24. 2% uranyl acetate (UA) (EMS, catalog number: 22400-2)
25. Ethane (C2H6) (Airgas, catalog number: ET RP35)
26. Liquid nitrogen (LN2) (Airgas, catalog number: NI UHP230LT350)
Solutions
1. Kanamycin stock solution, 1,000× (40 mg/mL) (see Recipes)
2. Kanamycin stock solution, 1,000× (90 mg/mL) (see Recipes)
3. Conditioning plate (see Recipes)
4. Pre-growth media (see Recipes)
5. Lysis buffer (see Recipes)
6. Wash buffer (see Recipes)
7. Growth media (see Recipes)
8. IPTG stock solution (see Recipes)
9. Buffer A (see Recipes)
10. Buffer B (see Recipes)
11. TEN buffer (see Recipes)
12. Saturated ammonium sulfate solution (see Recipes)
13. Fibrilization buffer (see Recipes)
14. 1% uranyl acetate (UA) (see Recipes)
Recipes
1. Kanamycin stock solution, 1,000× (40 mg/mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Kanamycin monosulfate | 40 mg/mL | 0.4 g |
| 2H2O | n/a | 10 mL |
| Total | n/a | 10 mL |
a. Completely dissolve kanamycin monosulfate in 2H2O.
b. Sterilize solution using a 0.22 μm syringe filter and 10 mL syringe.
c. Aliquot 1,000 μL stocks and store at -20 °C until use.
2. Kanamycin stock solution, 1,000× (90 mg/mL)
| Reagent | Final concentration | Amount |
|---|---|---|
| Kanamycin monosulfate | 90 mg/mL | 0.9 g |
| 2H2O | n/a | 10 mL |
| Total | n/a | 10 mL |
a. Completely dissolve kanamycin monosulfate in 2H2O.
b. Sterilize solution using a 0.22 μm syringe filter and 10 mL syringe.
c. Aliquot 1,000 μL stocks and store at -20 °C until use.
3. Conditioning plate
| Reagent | Final concentration | Amount |
|---|---|---|
| 2H2O | 70% | 700 mL |
| LB broth | 2% | 20 g |
| Bacto agar | 1.5% | 15 g |
| H2O | n/a | Fill to 1,000 mL |
| Total | n/a | 1,000 mL |
a. Combine reagents in a flask and autoclave at 121 °C, 15 psi for at least 20 min.
b. Allow the media to cool to ~55 °C, then add 1,000 μL of the kanamycin stock solution (1,000×, 40 mg/mL).
c. Pour ~25 mL of media per Petri plate (100 mm) and repeat for the remaining 1 L.
4. Pre-growth media
| Reagent | Final concentration | Amount |
|---|---|---|
| 2H2O | 70% | 35 mL |
| LB broth | 2% | 1 g |
| H2O | n/a | Fill to 50 mL |
| Total | n/a | 50 mL |
a. Combine reagents in a flask and autoclave at 121 °C, 15 psi for at least 20 min.
b. Allow the media to cool to ~55 °C and then add 50 μL of the kanamycin stock solution (1,000×, 40 mg/mL).
5. Lysis buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| NaOH | 40 mM | 0.80 g |
| Tris-HCl | 20 mM | 3.15 g |
| EDTA | 1 mM | 0.0585 mg |
| Triton X-100 | 0.1% (v/v) | 0.5 mL |
| Total | n/a | 500 mL |
Combine reagents and adjust pH to 8.0 with NaOH.
6. Wash buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| NaH2PO4 | 50 mM | 0.34 g |
| KH2PO4 | 25 mM | 0.17 g |
| NaCl | 10 mM | 0.03 g |
| 2H2O | n/a | Fill to 50 mL |
| Total | n/a | 50 mL |
a. Combine reagents and adjust the pH to 7.6 with NaO2H.
b. Filter sterilize the solution using a 500 mL filtration system.
7. Growth media
| Reagent | Final concentration | Amount |
|---|---|---|
| NaH2PO4 | 50 mM | 6.9 g |
| KH2PO4 | 25 mM | 3.4 g |
| NaCl | 10 mM | 0.58 g |
| MgSO4 | 5 mM | 1.23 g |
| CaCl2 | 0.2 mM | 0.03 g |
| Bacterial cell media 10× | 0.1× | 10 mL |
| BME vitamins 100× | 0.25× | 2.5 mL |
| Studier trace metals 1,000× | 0.25× | 0.25 mL |
| 15N-NH4CI | 1 g/L | 1 g |
| 2H-13C-glucose | 8 g/L | 8 g |
| BME vitamins | 0.25× | 2.5 mL |
| 2H2O | n/a | Fill to 50 mL |
| Total | n/a | 1,000 mL |
a. Combine reagents and add 1,000 μL of the kanamycin stock solution (1,000×, 90 mg/mL). Adjust pH to 7.6 with NaO2H.
b. Filter sterilize the solution using a 1,000 mL filtration system.
8. IPTG stock solution
| Reagent | Final concentration | Amount |
|---|---|---|
| IPTG | 0.5 M | 1.2 g |
| 2H2O | n/a | 10 mL |
| Total | n/a | 10 mL |
a. Completely dissolve IPTG in 2H2O.
b. Sterilize solution using a 0.22 μm syringe filter and 10 mL syringe.
c. Aliquot 1,000 μL stocks and store at -20 °C until use.
9. Buffer A
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris-HCl | 30 mM | 4.73 g |
| NaCl | 30 mM | 1.75 g |
| H2O | n/a | 1 L |
| Total | n/a | 1 L |
a. Dissolve Tris-HCl and NaCl in water while stirring.
b. Adjust pH to 7.4 at 37 °C using 1 M NaOH.
10. Buffer B
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris-HCl | 30 mM | 2.36 g |
| NaCl | 1 M | 29.22 g |
| H2O | n/a | 500 mL |
| Total | n/a | 500 mL |
a. Dissolve Tris-HCl and NaCl in water while stirring.
b. Adjust pH to 7.4 at 37 °C using 1 M NaOH.
11. TEN buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| Tris-HCl | 30 mM | 4.73 g |
| NaCl | 30 mM | 1.75 g |
| EDTA | 0.1 mM | 29.22 mg |
| H2O | n/a | 1 L |
| Total | n/a | 1 L |
a. Dissolve Tris-HCl, NaCl, and EDTA in water while stirring.
b. Adjust pH to 8.0 at 37 °C using 1 M NaOH.
12. Saturated ammonium sulfate solution
| Reagent | Final concentration | Amount |
|---|---|---|
| Ammonium sulfate | saturated | ~550 g |
| H2O | n/a | 1 L |
| Total | n/a | 1 L |
a. Add ammonium sulfate into water while stirring.
b. Heat gently until all ammonium sulfate is dissolved.
c. Cool to room temperature. Crystals should form to indicate the solution is saturated.
13. Fibrilization buffer
| Reagent | Final concentration | Amount |
|---|---|---|
| NaH2PO4 | 50 mM | 1.2 g |
| EDTA | 0.1 mM | 5.85 mg |
| Sodium azide | 0.02% | 40 µg |
| H2O | 20% | 40 mL |
| 2H2O | 80% | 160 mL |
| Total | n/a | 200 mL |
a. Add NaH2PO4, EDTA, and 0.02% sodium azide solution into 2H2O and H2O.
b. Adjust pH to 7.4 at 37 °C using 1M NaO2H.
14. 1% uranyl acetate (UA)
| Reagent | Final concentration | Amount |
|---|---|---|
| 2% uranyl acetate | 1% (v/v) | 250 μL |
| H2O | n/a | 250 μL |
| Total | n/a | 500 μL |
Mix 1 part of sterile water with 1 part of 2% UA and filter through a Spin-X centrifuge tube with a 0.22 μm filter.
Laboratory supplies
1. 10 mL syringe (BD, catalog number: 309604)
2. 0.22 μm filter (GenClone, catalog number: 25-240)
3. 100 mm × 15 mm Petri dishes (Fisher Scientific, catalog number: S33580A)
4. 500 mL filtration system (Nalgene, catalog number: 595-4520)
5. 1,000 mL filtration system (Fisher Scientific, catalog number: FB12566506)
6. 50 mL conical tubes (VWR, catalog number: 525-1074)
7. 0.45 μm syringe filter (GenClone, catalog number: 25-246)
8. 1.7 mL centrifuge tubes (Denville, catalog number: C2170)
9. Parafilm (Bemis, catalog number: PM996)
10. 0.22 μm Spin-X centrifuge tube filter (Costar, catalog number: 8160)
11. 200 mesh carbon film, copper grids (EMS, catalog number: CF200-CU)
12. Whatman #1 filter paper (Whatman, catalog number: 1001-090)
13. Quantifoil R2/1 200 mesh, copper grids (Quantifoil Micro Tools GmbH, catalog number: Q210CR1)
14. Standard Vitrobot filter paper, Ø 55/20 mm, grade 595 (Ted Pella, catalog number: 47000-100)
Equipment
1. HiTrap Q HP anion exchange column with QFF anion exchange resin (Cytiva, catalog number: 17115401)
2. Stirred cell concentrator (Amicon, catalog number: UFSC05001)
3. Ultracel 3 kDa ultrafiltration disc (Amicon, catalog number: PLBC04310)
4. HiPrep 16/60 Sephacryl S100-HR gel filtration column (Cytiva, catalog number: 17119501)
5. 5424 R microcentrifuge (Eppendorf, catalog number: 05-400-005)
6. 5810 R centrifuge (Eppendorf, catalog number: 022625101)
7. J6-MI high-capacity centrifuge (Beckman Coulter, catalog number: 449598)
8. 1 L centrifuge bottle (Beckman Coulter, catalog number: C31597)
9. myTemp digital incubator (Benchmark Scientific, catalog number: H2200-HC)
10. Pyrex 250 mL Erlenmeyer flask (Corning, catalog number: 4980-250)
11. Floor orbital shaker (Thermo Electron Corporation, catalog number: 19141, model: 480)
12. Denovix UV spectrophotometer/fluorimeter (Denovix, model: DS-11 FX+)
13. Quartz cuvette (Denovix, catalog number: A-70031)
14. ӒKTA pure 25 M (Cytiva, catalog number: 29018226)
15. Grid holder block (Pelco, catalog number: 16820-25)
16. Plasma cleaner (Harrick Plasma Inc., catalog number: PDC-32G)
17. Static dissipator (Mettler Toledo, catalog number: UX-11337-99)
18. Style N5 reverse pressure tweezers (Dumont, catalog number: 0202-N5-PS-1)
19. Talos L120C 120 kV transmission electron microscope (TEM) (Thermo Fisher Scientific or equivalent)
20. Cryo grid box (Sub-Angstrom, catalog number: SB)
21. Vitrobot Mark IV vitrification robot (Thermo Fisher Scientific)
22. Titan Krios G3i 300 kV transmission electron microscope (TEM) (Thermo Fisher Scientific)
23. K3-GIF direct electron detector with energy filter (Gatan Inc., AMETEK)
24. High-performance computing (HPC) cluster with an EPYC Milan 7713P 64-core 2.0 GHz CPU (AMD), 512 GB RAM, 4× RTX A5000 24 GB GDDR6 GPU (NVIDIA), 2× 960 GB Enterprise SSD, mirrored OS, 2× 7.68 TB nVME SSD as 15 TB scratch space, dual-port 25 GbE Ethernet
Software and datasets
1. EPU/AFIS (https://thermofisher.com/smart-epu)
2. SBGrid (https://sbgrid.org/) [34]
3. IMOD (https://bio3d.colorado.edu/imod/) [35]
4. RELION (https://relion.readthedocs.io/en/release-4.0/) [36,37]
5. MotionCor2 (https://emcore.ucsf.edu/ucsf-software) [38]
6. Gctf (https://sbgrid.org/software/titles/gctf) [39]
7. Topaz-filament (https://github.com/3dem/topaz) [40]
8. UCSF ChimeraX (https://www.cgl.ucsf.edu/chimerax/) [41,42]
9. Coot (https://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/)
10. PHENIX (https://phenix-online.org/) [43]
Procedure
文章信息
稿件历史记录
提交日期: Aug 18, 2024
接收日期: Nov 28, 2024
在线发布日期: Dec 17, 2024
出版日期: Feb 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Sanchez, J. C., Pierson, J. A., Borcik, C. G., Rienstra, C. M. and Wright, E. R. (2025). High-resolution Cryo-EM Structure Determination of a-Synuclein—A Prototypical Amyloid Fibril. Bio-protocol 15(3): e5171. DOI: 10.21769/BioProtoc.5171.
分类
生物物理学 > 显微技术 > 低温显微镜技术
生物化学 > 蛋白质 > 成像
生物物理学 > 电子冷冻断层扫描
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













