- EN - English
- CN - 中文
In Vitro Assay to Examine Osteoclast Resorptive Activity Under Estrogen Withdrawal
体外检测雌激素剔除条件下破骨细胞吸收活性的实验方法
(*contributed equally to this work) 发布: 2025年01月05日第15卷第1期 DOI: 10.21769/BioProtoc.5155 浏览次数: 1662
评审: Athanas GuzhaWei DaiJaira Ferreira de VasconcellosAnonymous reviewer(s)
Abstract
The bone is a highly dynamic organ that undergoes continuous remodeling through an intricate balance of bone formation and degradation. Hyperactivation of the bone-degrading cells, the osteoclasts (OCs), occurs in disease conditions and hormonal changes in females, resulting in osteoporosis, a disease characterized by altered microarchitecture of the bone tissue, and increased bone fragility. Thus, building robust assays to quantify OC resorptive activity to examine the molecular mechanisms underlying bone degradation is critical. Here, we establish an in vitro model to investigate the effect of estrogen withdrawal on OCs derived from the mouse macrophage RAW 264.7 cell line in a bone biomimetic microenvironment. This simple and robust model can also be adapted to examine the effect of drugs and genetic factors influencing OC resorptive activity in addition to being compatible with fluorescent imaging.
Key features
• A robust in vitro protocol that allows molecular and functional studies of mature osteoclasts in response to estrogen and its withdrawal.
• Generation of inorganic bone-mimetic substrates for culturing and examining osteoclast resorptive behavior.
• This quantitative image-based approach is compatible with brightfield and fluorescence microscopy to assess osteoclast resorptive activity.
Keywords: Estrogen withdrawal (雌激素剔除)Graphical overview
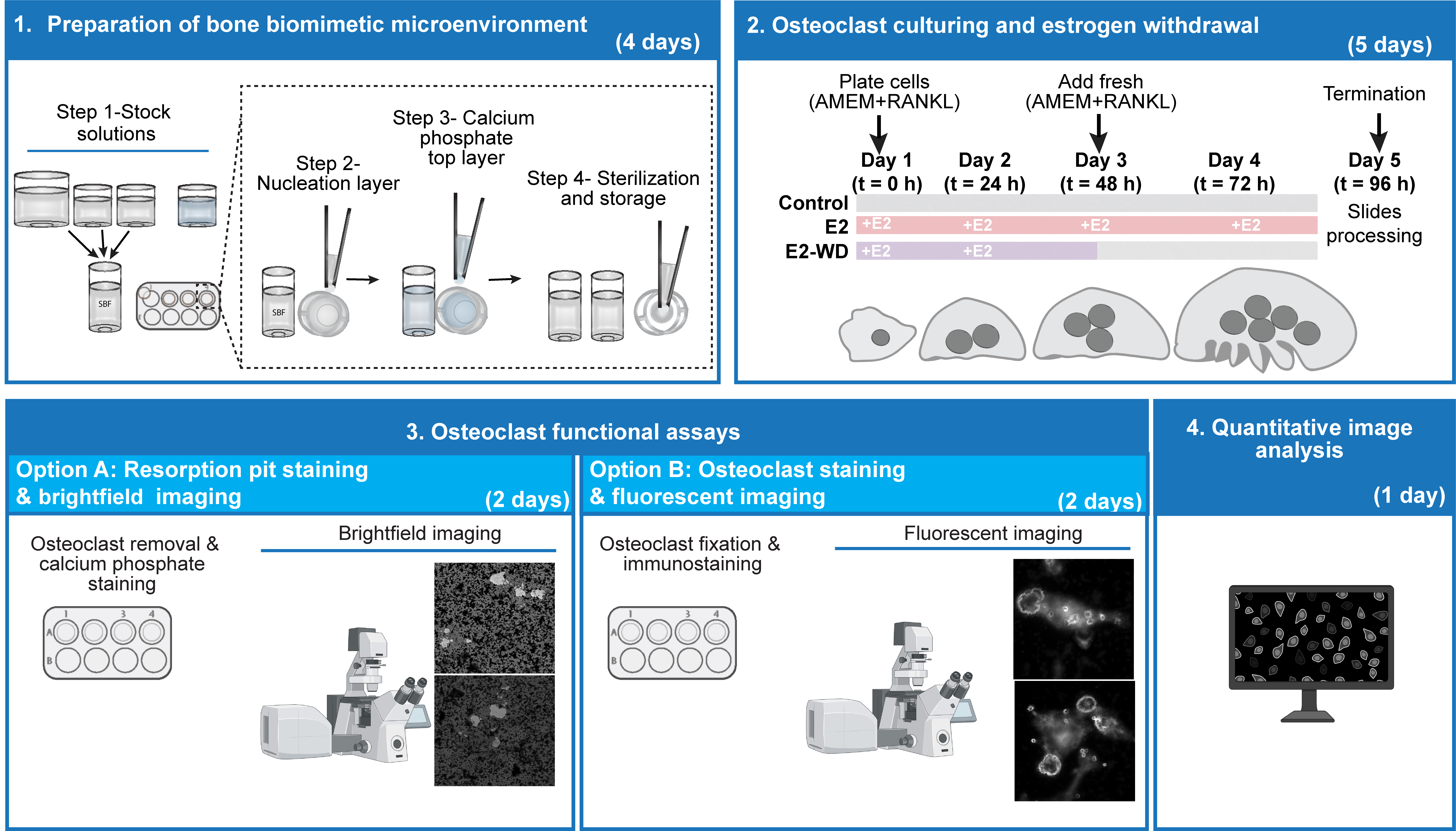
Background
Osteoclasts (OCs) are specialized, multinucleated cells responsible for the selective resorption of bone matrix [1,2]. In vivo osteoclast-driven resorption is followed by osteoblast (OB) matrix deposition, resulting in the continuous remodeling of bone [2,3]. The coordination between OC and OB activation relies on signaling from mechano-sensing osteocytes [2,3]. Disruption in this dynamic equilibrium produces disease states like osteoporosis [4,5]. Postmenopausal estrogen deficiency is a disrupting factor that shifts this equilibrium toward unregulated OC activity [3,6,7].
Estrogen (E2) deficiency de-regulates bone homeostasis; OB activity and lifespan are impaired, while increased osteoclast formation, resorptive activity, and lifespan contribute to net bone loss [3,7]. The effects of estrogen loss on OC formation and function have previously been studied using ovariectomized mouse models and bone marrow–derived osteoclasts in vitro [8,9]. Primary macrophages and OCs are challenging to work with and place limits on the biochemical and molecular analyses one can perform [10]. In vitro models of estrogen deficiency using OB and osteocyte cell lines exist [11–13]; however, this protocol establishes a simple and reproducible in vitro system to examine the effect of estrogen presence and withdrawal on OCs [14]. In contrast to existing models, using the murine RAW 264.7 cell line produces a large and consistent source of OCs, avoiding complicated extraction and propagation in favor of greater research output. This simple, robust method is a powerful tool to study osteoclast activation and function under postmenopausal conditions.
To measure OC activity, resorptive capacity can be assayed using coverslips coated with a thin layer of inorganic calcium phosphate. Maria et al. [15] thoroughly characterized and validated biomimetic calcium phosphate coating to study OC activity. Our protocol outlines the production of the coating and includes the culturing, staining, and subsequent imaging and analysis that can be performed. We provide this method as an alternative to using standard animal bone and dentine slices [16,17]. While suitable for resorption pit studies, bone and dentine slices do not allow for easy visualization of OCs remaining on the substrate [18]. In addition to quantifying OC resorptive activity, our lab has used biomimetic bone-coated coverslips combined with immunofluorescence to examine cytoskeletal elements, including F-actin ring morphology, the microtubule network, and centrosome de-clustering through inhibitor studies [14,19].
Materials and reagents
Biological materials
1. RAW 264.7 mouse macrophage cell line (American Type Culture Collection, catalog number: TIB-71)
Note: To maintain consistency across replicates, we recommend using RAW cells between passages 3 and 13. We have observed reduced OC formation in older passages.
Reagents
A. Bone mimetic production and staining reagents
1. Tris base (BioShop, catalog number: TRS001)
2. Hydrochloric acid solution, 1 N (HCl) (Thermo Fisher Scientific, catalog number: FLSA481)
3. Sodium hydroxide solution, 1 N (NaOH) (Thermo Fisher Scientific, catalog number: 124260010)
4. Calcium chloride dihydrate (CaCl2·2H2O) (BioShop, catalog number: CCL302)
5. Sodium chloride (NaCl) (BioShop, catalog number: CCL302)
6. Magnesium chloride hexahydrate (MgCl2·6H2O) (BioShop, catalog number: MAG510)
7. Disodium hydrogen phosphate dihydrate (Na2HPO4·2H2O) (MilliporeSigma, catalog number: 71643)
8. Sodium bicarbonate (NaHCO3) (BioShop, catalog number: SOB999)
9. Distilled water; provided by facility
10. Milli-Q water
11. 70% ethanol prepared from anhydrous or 95% ethyl alcohol
12. Sodium nitrate solution, 2.5% (AgNO3) (MilliporeSigma, catalog number: 85193)
13. Triton X-100 (BioShop, catalog number: TRX506)
B. Cell culture reagents
1. Dulbecco’s modified Eagle medium (DMEM) (Wisent Inc., catalog number: 319-005-CL)
2. Fetal bovine serum (FBS) (Wisent Inc., catalog number: 085-450)
3. Phosphate-buffered saline (PBS) (Wisent Inc., catalog number: 311-010-CL)
C. OC differentiation reagents
1. Alpha modified Eagle medium (AMEM) (Thermo Fisher Scientific, catalog number: 12571063)
2. 17β-estradiol (MilliporeSigma, catalog number: E8875)
3. Dimethyl sulfoxide (DMSO) (BioShop, catalog number: DMS555.250)
4. Recombinant receptor activator of nuclear factor kappa-B ligand (RANKL) was produced from BL21 Escherichia coli transformed with a pGEX-GST-hRANKL vector (a gift from Morris Manolson, Faculty of Dentistry, University of Toronto).
Commercial alternative: Mouse TRANCE recombinant protein (Thermo Fisher Scientific, catalog number: 315-11-10UG)
D. Osteoclast fixation and staining reagents
1. Paraformaldehyde (PFA) (Electron Microscopy, catalog number: 15710-S)
2. Permeabilization buffer (0.1% Triton X-100, 100 mM glycine in PBS) (BioShop, catalog number: GLN001)
3. Alexa Fluor 488 Phalloidin (Thermo Fisher Scientific, catalog number: 12379)
4. DAPI (Thermo Fisher Scientific, catalog number: D1306)
Solutions
Solutions required to prepare bone mimetic:
1. 50 mM Tris base stock solution, pH 7.4 (see Recipes)
2. Calcium stock solution (see Recipes)
3. Phosphate stock solution (see Recipes)
4. Calcium phosphate solution (see Recipes)
5. Simulated body fluid solution (SBF) (see Recipes)
6. Cell removal solution (see Recipes)
Recipes
1. Tris base stock solution (500 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base | 50 mM | 3.03 g |
| 1 N HCl | n/a | see note* |
| Milli-Q water | n/a | see note* |
| Total | n/a | 500 mL |
*Note: Add Tris base to 400 mL of Milli-Q water and 20 mL of 1 N HCl. Once dissolved, bring pH to 7.4 by slowly adding 0.05 mL increments of 1 N HCl. Bring the final buffer volume to 500 mL using Milli-Q water.
Store at room temperature for 1 month.
2. Calcium stock solution (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| CaCl2·2H2O | 25 mM | 0.37 g |
| NaCl | 1.37 M | 8.00 g |
| MgCl2·6H2O | 15 mM | 0.30 g |
| Tris base stock solution | 50 mM | see note* |
| Total | n/a | 100 mL |
*Note: Add reagents to 90 mL of Tris base stock solution. Once dissolved, measure pH. Adjust to pH 7.38–7.4, if required, by slowly adding 0.05 mL increments of 1 N HCl or 1 N NaOH. Bring the final volume to 100 mL using Tris base stock solution.
Store at room temperature for 1 month.
3. Phosphate stock solution (100 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4·2H2O | 11.1 mM | 0.20 g |
| NaHCO3 | 42 mM | 0.35 g |
| Tris base stock solution | 50 mM | see note* |
| Total | n/a | 100 mL |
*Note: Add reagents to 90 mL of Tris base stock solution. Once dissolved, measure pH. Adjust to pH 7.38–7.4, if required, by slowly adding 0.05 mL increments of 1 N HCl or 1 N NaOH. Bring the final volume to 100 mL using Tris base stock solution.
Store at room temperature for 1 month.
4. Calcium phosphate solution (250 mL)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Na2HPO4·2H2O | 2.25 mM | 0.10 g |
| CaCl2·2H2O | 4 mM | 0.15 g |
| NaCl | 0.14 M | 2.05 g |
| Tris base | 50 mM | 1.51 g |
| 1 N HCl | n/a | see note* |
| Milli-Q water | n/a | see note* |
| Total | n/a | 250 mL |
*Note: Add reagents to 200 mL of Milli-Q water and 10 mL of 1 N HCl. Once dissolved, bring pH to 7.4 by slowly adding 0.05 mL increments of 1 N HCl. Bring the final buffer volume to 250 mL.
Store at room temperature for 1 month.
5. Simulated body fluid solution (SBF)
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| Tris base stock solution | n/a | 25 mL |
| Calcium stock solution | n/a | 12.5 mL |
| Phosphate stock solution | n/a | 12.5 mL |
| Total | n/a | 50 mL; see note* |
*Note: Make SBF immediately before adding to coverslips/wells. Maintain a ratio of 2:1:1 for Tris base:calcium:phosphate stock solutions and adjust the total volume as required for your experiment.
Make fresh and use immediately.
6. Cell removal solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| NaCl | 1 M | 5.84 g |
| Triton X-100 | 0.20 % | 0.2 mL; see note* |
| Distilled water | n/a | 100 mL |
| Total | n/a | see note* |
*Note: Prepare 1 M NaCl before adding the Triton X-100. Sterile-filtered 1 M NaCl can be prepared separately and stored at room temperature for 1 month.
Make fresh and use immediately.
Laboratory supplies
1. 12-well 18R glass coverslips (Electron Microscopy Sciences, catalog number: 72290-08)
2. 12-well tissue culture plates (Sarstedt, catalog number: 83.3921)
3. Parafilm (MilliporeSigma, catalog number: P7668)
4. 0.22 μm sterile bottle top filter (Sarstedt, catalog number: 83.3941.511)
5. T-75 cell culture flasks (Sarstedt, catalog number: 83.3911.002)
6. 5 and 10 mL disposable serological pipettes (Sarstedt, catalog number: 86.1253.001 and 86.1254.001, respectively)
7. 10, 200, 1,000 μL standard micropipette disposable tips
8. 1.5 mL standard microcentrifuge tubes
9. Cell scrapers (Sarstedt, catalog number: 83.3951)
10. 15 mL conical tubes (Sarstedt, catalog number: 62.554.205)
11. Microscope slides (Thermo Fisher Scientific, catalog number: 22037294)
12. DAKO (Agilent Technologies, catalog number: S302380-2)
13. Zeiss Immersion Oil 518F (Thermo Fisher Scientific, catalog number: 12-624-66A)
14. Slide folder (Thermo Fisher Scientific, catalog number: 12-587-10)
Equipment
1. Analytical balance (Mettler Toledo, catalog number: MS204S)
2. Magnetic stir plate (Thermo Fisher Scientific, catalog number: 1160049H)
3. 37 °C non-humidified incubator (Thermo Fisher Scientific, model: 637D)
4. 37 °C, 5% CO2 humidified incubator (Thermo Electron Corporation, model: HERACELL VIOS 160i)
5. 37 °C water bath (Thermo Fisher Scientific, model: ISOTEMP 110)
6. Vacuum aspirator (Integra-biosciences, catalog number: 158320)
7. Pipetman 2, 20, 200, and 1,000 μL (Gilson)
8. Autoclave; provided by research facility
9. Milli-Q Integral Water Purification System supplemented with Millipak® Express 0.22 μm membrane filter (Millipore, catalog number: MPGP04001)
10. pH meter (Thermo Fisher Scientific, model: AB150)
11. 500 and 1,000 mL glass beakers
12. 50 and 500 mL graduated cylinders
13. 250 and 500 mL glass bottles
14. Brightline hemacytometer (Electron Microscopy Sciences, catalog number: 100498-504)
15. Fine tip tweezers (Excelta, catalog number: 3C-SA-SE)
16. Inverted epifluorescence microscope with differential interference contrast (DIC) at 20× and 40× (Zeiss, model: AxioObserver Z1)
17. Inverted spinning disk confocal microscope with DIC at 40× 1.4 NA oil immersion lens (Quorum Technologies Inc., model: Quorum WaveFX-X1)
18. BSL-2 biosafety cabinet (Thermo Fisher Scientific, model: 1375 Series Class II)
Software and datasets
1. Zeiss Zen (3.1, blue edition, June 2012); license required
2. MetaMorph; license required
3. Fiji/ImageJ (2.3.0, May 2017); freely available
4. Prism GraphPad Software (7.03, March 2017); license required
Procedure
文章信息
稿件历史记录
提交日期: Aug 21, 2024
接收日期: Nov 3, 2024
在线发布日期: Nov 20, 2024
出版日期: Jan 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Fiorino, C., Omer, S., Gandhi, N. and Harrison, R. E. (2025). In Vitro Assay to Examine Osteoclast Resorptive Activity Under Estrogen Withdrawal. Bio-protocol 15(1): e5155. DOI: 10.21769/BioProtoc.5155.
分类
细胞生物学 > 基于细胞的分析方法 > 胞外微环境
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link