- EN - English
- CN - 中文
Isolation of Viral Biofilms From HTLV-1 Chronically Infected T Cells and Integrity Analysis
从慢性感染HTLV-1的T细胞中分离病毒生物膜及其完整性分析
发布: 2024年12月20日第14卷第24期 DOI: 10.21769/BioProtoc.5152 浏览次数: 1812
评审: Alka MehraJibin SadasivanSrajan KapoorAnonymous reviewer(s)
Abstract
The human T-lymphotropic virus type-1 (HTLV-1) is an oncogenic retrovirus that predominantly spreads through cell-to-cell contact due to the limited infectivity of cell-free viruses. Among various modes of intercellular transmission, HTLV-1 biofilms emerge as adhesive structures, polarized at the cell surface, which encapsulate virions within a protective matrix. This biofilm is supposed to facilitate simultaneous virion delivery during infection. Yet, the molecular and functional intricacies of viral biofilms remain largely unexplored, despite their pivotal role in understanding retroviral pathogenesis. In this study, we optimized a protocol to isolate HTLV-1 biofilms from chronically infected T cells, facilitating their structural and molecular characterization using proteomic and super-resolution microscopy analyses. This protocol involves cultivating HTLV-1 chronically infected T cells at high density to facilitate the natural detachment of viral biofilms into the supernatant. Then, employing successive centrifugations, the cells are separated from the detached biofilms, and these structures are pelleted at medium speed (10,000× g). This method circumvents the need for mechanical, chemical, or enzymatic biofilm detachment, bypasses the use of ultracentrifugation, and enables us to resuspend the biofilms in the appropriate buffer for subsequent analyses such as western blotting or super-resolution microscopy imaging as presented.
Key features
• Isolation of viral biofilms from HTLV-1 chronically infected T cells after 4 days of culture at high cellular density.
• Structural analysis of viral biofilms using super-resolution microscopy techniques.
• Experiments performed in vitro within a confined biosafety level 3 (BSL3) environment.
• This protocol requires at least five days to complete.
Keywords: HTLV-1 (HTLV-1)Graphical overview
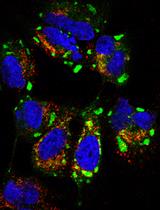
Fluorescent HTLV-1 viruses embedded in a biofilm
Background
The human T-lymphotropic virus type-I (HTLV-1) is an oncogenic retrovirus estimated to infect 5–10 million people worldwide [1]. Infected cells are characterized by the integration of the reverse-transcribed viral genome (proviral DNA) in the host genome, which can be expressed using the host transcription machinery, generating new virions and thus leading to chronic infection. Although most people remain asymptomatic following HTLV-1 exposure, chronic infections can lead to aggressive pathologies such as adult T-cell leukemia [2] or progressive inflammatory disorders like HTLV-1-associated myelopathy [3]. In vivo, HTLV-1 is primarily detected in CD4(+) T cells, and its dissemination among individuals occurs through three main routes: mother to child during breastfeeding, sexual contact, or exposure to HTLV-1-infected blood products [4]. Overall, this virus represents a major health issue as no existing strategy allows the efficient protection of exposed individuals. Therefore, fundamental research on HTLV-1 dissemination routes is essential to promote the expansion of pertinent therapeutic approaches.
Unlike most retroviruses, evidence strongly suggests that HTLV-1 cell-free virions are poorly infectious in vivo [5,6]. As such, HTLV-1 relies mostly on intercellular contacts to disseminate within its host. Three cell-to-cell transmission modes have been described: virological synapses, cellular conduits, and viral biofilms, which are of interest here [7]. Viral biofilms are cell-surface aggregates of viruses embedded in extracellular matrix components that accumulate virions at one or several poles of the infected T cells [8]. These structures allow the bulk delivery of infectious particles to target cells and may help to protect HTLV-1 virions from immune attacks [9,10]. While the relative importance of the different cell-to-cell transmission routes is difficult to ascertain in vivo, the removal of biofilms by heparin washes reduces the infectious capacity of HTLV-1-producing cells by 80% in vitro [9]. In addition, biofilms detached from HTLV-1-infected cells are infectious in vitro, as opposed to cell-free virions released in the supernatant of the same cell population [9,11], indicating that the transfer of HTLV-1 biofilms through cell-to-cell contact is the most efficient pathway to infect new cells.
To identify molecular factors involved in HTLV-1 biofilm architecture and explore their impact on viral transmission, we sought to isolate these structures, analyze their molecular composition using mass spectrometry, and confirm by super-resolution STED (stimulated-emission-depletion) microscopy [12]. Here, we will detail the protocol of biofilm isolation that we used in Arone et al. [13] and the techniques employed to validate our protocol (i.e., western blotting and STED microscopy). This protocol is an optimized version of procedures published in Pais-Correia et al. [9] and Alais et al. [11]. In these studies, authors used chemical or mechanical methods, respectively, to detach viral biofilms from HTLV-1 chronically infected T cells and used ultracentrifugation to collect them. While our protocol is also based on cell culture at high density to enrich viral biofilms in the supernatant, it circumvents the need for mechanical or chemical biofilm detachment and bypasses the use of ultracentrifugation. Finally, using immuno-staining coupled with super-resolution STED microscopy techniques [13], we confirm that our isolation protocol preserves the integrity of viral biofilms that contain unaltered virions.
Materials and reagents
Biological materials
HTLV-1 chronically infected T cells (C91-PL) derived from umbilical cord blood cells (CVCL_0197, https://www.cellosaurus.org/CVCL_0197).
pHTLV Gag-YFP plasmid was described previously in Heidecker et al. [14] and is a kind gift from former Dr. Derse’s lab.
Phosphate buffered saline (PBS) pH 7,4 (Gibco, catalog number: 11503387)
Roswell Park Memorial Institute (RPMI) medium (Gibco, catalog number: 11875093)
Opti-MEM reduced serum medium (Gibco, catalog number: 31985062)
Fetal calf serum (FCS) (Sigma-Aldrich, catalog number: F7524)
Penicillin-streptomycin (Sigma-Aldrich, catalog number: P4333)
Trypan blue stain 0.4% (Logos biosystems, catalog number: T13001)
Poly-L-lysine hydrobromide, 5 mg (Sigma-Aldrich, catalog number: P6282)
Bovine serum albumin (BSA) lyophilized powder (Sigma-Aldrich, catalog number: A9418)
32% paraformaldehyde (PFA) without methanol (Thermo Fisher Scientific, catalog number: 047377.9L)
Triton X-100 (Sigma-Aldrich, catalog number: X100)
NaCl 5M solution (Thermo Fisher Scientific, catalog number: AM9760G)
NaCl powder (Sigma-Aldrich, catalog number: S9888)
EDTA 0.5M pH 8.0 (Thermo Fisher Scientific, catalog number: 15575020)
40% acrylamide/bisacrylamide (Euromedex, catalog number: EU0063-C)
Saponin (Sigma-Aldrich, catalog number: SAE0073)
NH4Cl (Sigma-Aldrich, catalog number: A9434)
Methanol (Honeywell, catalog number: 14262-1L)
37% hydrochloric acid (HCl) solution (Honeywell, catalog number: 30721)
Trizma base, crystalline (Sigma-Aldrich, catalog number: T1503)
Tris-glycine 10× buffer (Euromedex, catalog number: EU0550)
Tris-glycine SDS 10× buffer (Euromedex, catalog number: EU0510)
Tris/HCl 1M pH 7.4 (Thermo Fisher Scientific, catalog number: J67501.AE)
10% ammonium persulfate (APS) (Thermo Fisher Scientific, catalog number: 17874)
TEMED (Sigma-Aldrich, catalog number: T9281)
Laemmli SDS 4× buffer (Thermo Fisher Scientific, catalog number: J60015.AC)
Sterile Braun water (Dutscher, catalog number: 921120)
PageRuler prestained protein ladder (Thermo Fisher Scientific, catalog number: 26616)
Tween 20 (Thermo Fisher Scientific, catalog number: 85115)
Milk powder (Régilait)
ECL prime western blotting detection reagent (Amersham, catalog number: 10308449)
Anti-HTLV-1 Gagp19 primary mouse antibody (Zeptometrix, catalog number: 801108)
Anti-HTLV-1 Envgp46 primary mouse antibody (Zeptometrix, catalog number: 0801127)
Anti-YFP primary rabbit antibody (Thermo Fisher Scientific, catalog number: A-11122)
Anti-mouse IgG STAR Red (Abberior, catalog number: 52283)
Anti-rabbit IgG STAR 580 (Abberior, catalog number: 41367)
Anti-mouse IgG HRP (Dako, catalog number: P0260)
TNE buffer (see Recipes)
Protein solubilization buffer (see Recipes)
Poly-L-lysine coating solution (see Recipes)
Fixation buffer (see Recipes)
Permeabilization and saturation buffer (see Recipes)
Tris-HCl 0.5M pH 6.8 (see Recipes)
Tris-HCl 1M pH 8.0 (see Recipes)
Tris-HCl 1.5M pH 8.8 (see Recipes)
Tris-buffered saline (TBS) (see Recipes)
Tris-buffered saline with Tween (TBST) (see Recipes)
10% separating electrophoresis gel (see Recipes)
4% stacking electrophoresis gel (see Recipes)
Western blot running buffer (see Recipes)
Western blot transfer buffer (see Recipes)
TNE buffer
Reagent Final concentration Quantity or Volume Tris/HCl 0.5M pH 7.4 10mM 200 μL NaCl 5M 100mM 400 μL EDTA 0.5M pH 8.0 1mM 40 μL Milli-Q H2O Up to 20 mL Total 20 mL Note: Store at room temperature.
Protein solubilization buffer
Reagent Final concentration Quantity or Volume Triton X-100 0.2% 2 mL PBS pH 7.4 99.8% 8 mL Total 100% 10 mL Note: Store at 4 °C.
Poly-L-lysine coating solution
Reagent Final concentration Quantity or Volume Poly-L-lysine hydrobromide 0.01% 5 mg PBS pH 7.4 99.9% Up to 50 mL Total 100% 50 mL Note: Store at -20 °C as 2 mL aliquots to avoid freeze/thaw cycles.
Fixation buffer
Reagent Final concentration Quantity or Volume 32% paraformaldehyde 4% 10 mL PBS pH 7.4 96% 70 mL Total 100% 80 mL Note: Store at -20 °C as 2 mL aliquots to avoid freeze/thaw cycles.
Permeabilization and saturation buffer
Reagent Final concentration Quantity or Volume Saponin 0.05% 5 mg BSA 3% 1.5 g PBS pH 7.4 96,95% Up to 50 mL Total 100% 50 mL Note: Mix thoroughly to homogenize completely. Use immediately.
Tris-HCl 0.5M pH 6.8
Reagent Final concentration Quantity or Volume Trizma base 30 g HCl (Adjust to pH 6.8) ~ 9.6 mL Milli-Q H2O Up to 500 mL Total 500 mL Note: HCl should be added in increments of 1–5 mL in 400 mL of H2O until the correct pH is reached. Allow the solution to mix completely before adding any more HCl. Complete to 500 mL with H2O. Store at room temperature.
Tris-HCl 1 M pH 8
Reagent Final concentration Quantity or Volume Trizma base 242 g HCl (Adjust to pH 8) ~ 50 mL Milli-Q H2O Up to 2 L Total 2 L Note: HCl should be added in increments of 1–5 mL in 1.6 L of H2O until the correct pH is reached. Allow the solution to mix completely before adding any more HCl. Complete to 500 mL with H2O. Store at room temperature.
Tris-HCl 1.5 M pH 8.8
Reagent Final concentration Quantity or Volume Trizma base 90.75 g HCl (Adjust to pH 8.8) ~ 9 mL Milli-Q H2O Up to 500 mL Total 500 mL Note: HCl should be added in increments of 1–5 mL in 400 mL of H2O until the correct pH is reached. Allow the solution to mix completely before adding any more HCl. Complete to 500 mL with H2O. Store at room temperature.
Tris-buffered saline (TBS) 10×
Reagent Final concentration Quantity or Volume Tris-HCl 1M pH 8 500 mL NaCl powder 7 g Milli-Q H2O Up to 1 L Total 1 L Note: Store at room temperature.
Tris-buffered saline supplemented with Tween (TBST) 1×
Reagent Final concentration Quantity or Volume TBS 10× 1× 100 mL Tween 20 1 mL Milli-Q H2O Up to 1 L Total 1 L Note: Store at room temperature.
10% separating electrophoresis gel
Reagent Final concentration Quantity or Volume 40% acrylamide/bisacrylamide 10% 2.5 mL Tris-HCl 1.5M pH 8.8 2.9 mL 10% APS 100 μL TEMED 10 μL Sterile Braun water 4.5 mL Total 10 mL (1 gel) Note: TEMED and APS should be added last to start gel polymerization. Add 500 μL of isopropanol on top of the gel to straighten it during polymerization. Use immediately.
4% stacking electrophoresis gel
Reagent Final concentration Quantity or Volume 40% acrylamide/bisacrylamide 4% 328 μL Tris-HCl 0.5M pH 6.8 830 μL 10% APS 25 μL TEMED 3.8 µL Milli-Q H2O 2.1 mL Total 3.3 mL (1 gel) Note: Remove the isopropanol covering the 10% gel. TEMED and APS should be added last to start gel polymerization. Use immediately.
Western blot running buffer
Reagent Final concentration Quantity or Volume Tris-glycine SDS 10× 1× 200 mL Milli-Q H2O 1.8 L Total 2 L Note: Store at room temperature.
Western blot transfer buffer
Reagent Final concentration Quantity or Volume Tris-glycine 10× 1× 200 mL Methanol 15% 300 mL Milli-Q H2O 1.5 L Total 2 L Note: Store at 4 °C.
Laboratory supplies
T25 cm3 cell culture flasks (Thermo Fisher Scientific, catalog number: 169900)
6-well plates (Thermo Fisher Scientific, catalog number: 10578911)
5 mL serological pipettes (Gilson, catalog number: F110126)
LUNA cell counting slides (Logos biosystems, catalog number: NC1765657)
1.5 mL sterile Eppendorf safe-lock tubes, microtube (Eppendorf, catalog number: 0030120086)
2 mL sterile Eppendorf safe-lock tubes, microtube (Eppendorf, catalog number: 0030120094)
5 mL sterile open-top thin wall ultra-clear tubes (Beckman Coulter, catalog number: C14279)
50 mL Falcon tubes (Thermo Fisher Scientific, catalog number: 10203001)
Filtered 1,000 μL pipette tips (Thermo Fisher Scientific, catalog number: 11749855)
Filtered 200 μL pipette tips (Thermo Fisher Scientific, catalog number: 11782584)
Filtered 10 μL pipette tips (Thermo Fisher Scientific, catalog number: 94052000)
Gene Pulser/MicroPulser electroporation cuvettes, 0.4 cm gap (Bio-Rad, catalog number: 1652081)
Coated glass bottom fluorodishes (WPI, catalog number: FD35-100)
Polyvinylidene difluoride membranes (Thermo Fisher Scientific, catalog number: 88518)
Electrophoresis gel wrap glass plates set (CBS Scientific, catalog number: MGP100R)
Whatman 3 mm paper for western blot (Cytiva, catalog number: 3630-917)
Equipment
Biosafety cabinet Herasafe KSP Class II (Thermo Fisher Scientific, model: 1300 series A2)
Incubator set to 5% CO2 and 37 °C (Binder, series BD)
ThermoMixer compact (Eppendorf, model: F1.5)
LUNA-II brightfield cell counter (Logos Biosystems, model: C100-Pro)
Eppendorf centrifuge with a fixed-angle rotor for 2 mL Eppendorfs (Thermo Fisher Scientific, model: 5430G)
Optima L-80 XP ultracentrifuge (Beckman Coulter, model: 392051)
FinnPip adjustable volume (P10, P200, P1000) pipettes (Thermo Fisher Scientific, model: monocanal Finnpip)
Pipette controller (Brandtech, model: accu-jet S)
Gene Pulser Xcell electroporation system (Bio-Rad, model: 1652660)
STED super-resolution microscope (Abberior Instruments, model: Expert Line GmbH)
ChemiDoc touch imaging system (Bio-Rad, model: 1708370)
Electrophoresis generator (Apelex, model: PS304 XL)
Double-wide mini-blotter (CBS Scientific, model: EBU-402)
Software and datasets
Image acquisition and analysis
Imspector Image Acquisition & Analysis Software (Abberior, v16.3, 2023)
ImageJ (Fiji, v1.54h, 15/12/2023)
Spectragryph (v1.2.16, 14/07/2022)
GraphPad Prism (v8.3.0, 28/10/2019)
Figure conception and formatting
Inkscape (v1.3.2, 26/11/2023)
Biorender (https://app.biorender.com/)
Procedure
文章信息
稿件历史记录
提交日期: May 4, 2024
接收日期: Oct 10, 2024
在线发布日期: Nov 18, 2024
出版日期: Dec 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Arone, C., Dutartre, H. and Muriaux, D. (2024). Isolation of Viral Biofilms From HTLV-1 Chronically Infected T Cells and Integrity Analysis. Bio-protocol 14(24): e5152. DOI: 10.21769/BioProtoc.5152.
分类
免疫学 > 免疫细胞染色 > 免疫检测
细胞生物学 > 细胞分离和培养 > 病毒分离
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link