- EN - English
- CN - 中文
An Assay System for Plate-based Detection of Endogenous Peptide:N-glycanase/NGLY1 Activity Using A Fluorescence-based Probe
基于荧光探针的酶标板内源性Peptide:N-糖苷酶/NGLY1活性检测系统
发布: 2025年01月05日第15卷第1期 DOI: 10.21769/BioProtoc.5151 浏览次数: 1529
评审: Laxmi Narayan MishraFNU PriyankaAnu ThomasAnonymous reviewer(s)
Abstract
Cytosolic peptide: N-glycanase (PNGase/NGLY1 in mammals), an amidase classified under EC:3.5.1.52, is a highly conserved enzyme across eukaryotes that catalyzes the removal of N-glycans from glycoproteins, converting N-glycosylated asparagine residues into aspartic acid. This enzyme also plays a role in the quality control system for nascent glycoproteins. Despite the development of non-radioisotope-based assay systems such as those using S-alkylated RNase or fluorescent-labeled glycopeptides as substrates, these methods are incompatible with crude enzyme sources, primarily due to the degradation of reaction products by contaminating endogenous proteases. We previously developed an assay system using a 5-carboxyfluorescein-labeled glycosylated cyclo-heptapeptide (5FAM-GCP), a substrate remarkably resistant to endogenous peptidase activity. This system enables the accurate measurement of endogenous NGLY1 activity in various samples, including cell lines, tissues, peripheral blood mononuclear cells, and NGLY1-deficient patient-derived cells, without the interference of proteolytic degradation. We recently advanced this approach by producing a novel fluorescence resonance energy transfer (FRET)-based GCP probe (fGCP) and demonstrated its ability to detect endogenous NGLY1 activity across diverse enzyme sources via fluorescence on multiarray plates. This innovative and straightforward assay now offers reliable disease diagnostics and also allows the measurement of endogenous PNGase/NGLY1 activities across various organisms.
Key features
• fGCP assay enables measurement of endogenous PNGase/NGLY1 activity in cells and tissues.
• An aliquot of 1–5 × 106 cells or 50–100 μg of protein extract from tissues is used for this assay.
• This assay enables microplate-based real-time measurement of endogenous PNGase/NGLY1 activities.
• This protocol requires a fluorescence plate reader equipped with an incubation function.
Keywords: Peptide:N-glycanase (Peptide:N-糖苷酶)Graphical overview
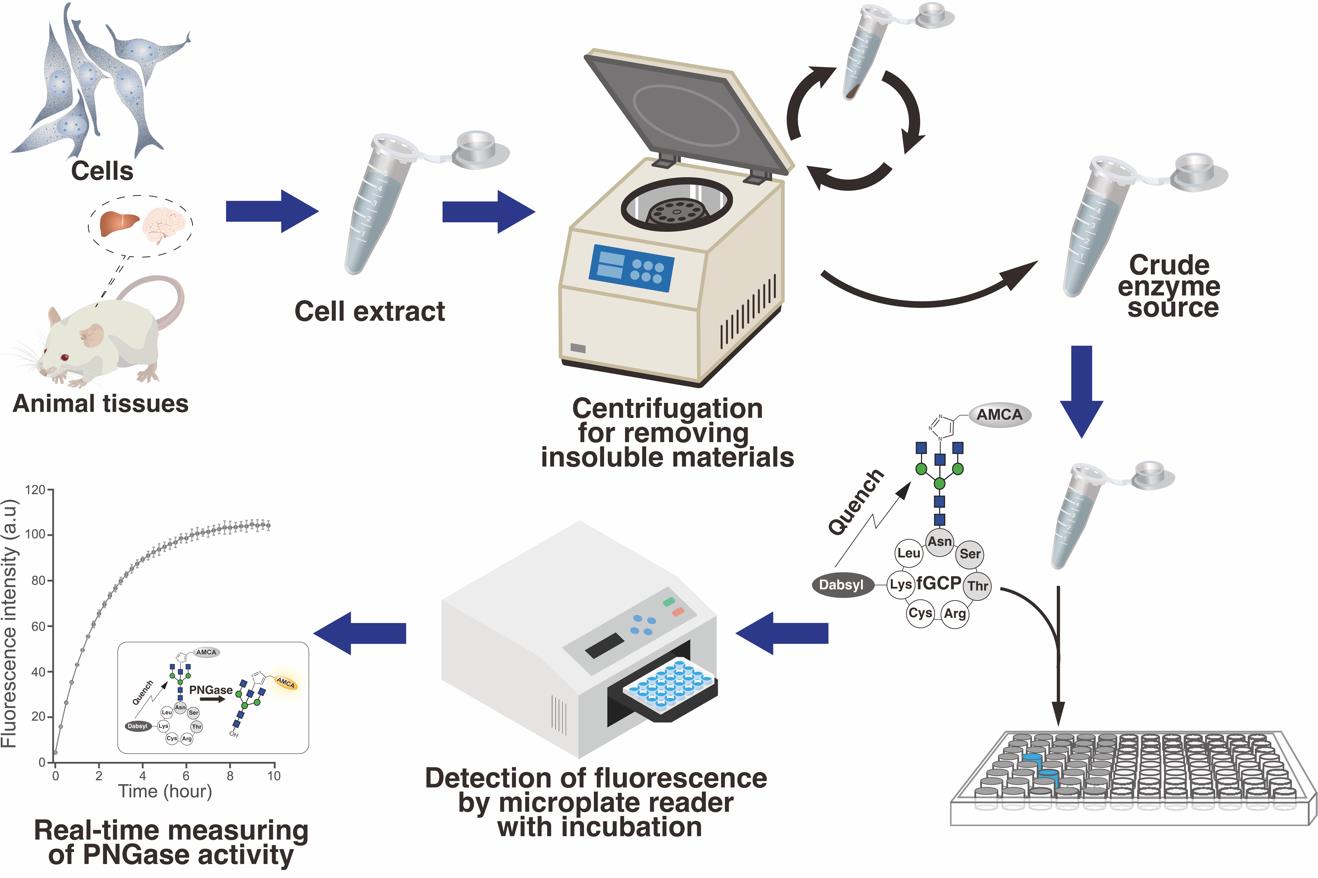
Overview of the real-time measurement of endogenous PNGase/NGLY1 activities in tissues/cells using fGCP assay
Background
Cytosolic peptide: N-glycanase (PNGase; NGLY1 in mammals) is an amidase that catalyzes the removal of N-glycans from the consensus sequences (Asn-Xaa-Ser/Thr, where Xaa represents any amino acid except proline) of glycoproteins, converting N-glycosylated Asn into Asp residues through deglycosylation [1]. Cytosolic PNGase also plays a role in the quality control system for newly synthesized glycoproteins [2].
To explore the biochemical properties of this enzyme, 14C-labeled glycopeptides, such as pentapeptides that carry asialoglycans derived from fetuin, have been commonly used as substrates for measuring PNGase activity [3]. Enzyme activity was measured based on the radioactivity of the reaction products, which were separated by paper chromatography or paper electrophoresis. However, the preparation of radioisotope-labeled glycopeptides and the use of radioactive molecules make it difficult to perform this assay in standard laboratory settings, especially under the tight regulations for the use of radioactive isotopes in Japan. Although non-radioisotope-based assays have been developed (e.g., assays using S-alkylated RNase [4] or fluorescent-labeled glycopeptides [5]), they are incompatible with crude enzyme sources due to the degradation of reaction products by contaminating endogenous proteases. Therefore, there exists a need to develop an alternative, easy-to-handle assay method.
An autosomal recessive disorder linked to NGLY1, known as NGLY1 deficiency or congenital disorder of deglycosylation (NGLY-CDDG) [OMIM: 615273], was first reported in 2012 [6]. Since then, more than 100 patients have been identified worldwide, including in Europe, America, Australia, India, China, and Japan [7,8]. The disease exhibits a broad spectrum of symptoms, including global developmental delay and/or intellectual disability, abnormal EEG, seizures, movement disorders, hypolacrima or alacrima, and liver dysfunction [8–13]. Unfortunately, there are no effective treatments; however, recent studies have demonstrated that administering an adeno-associated viral vector serotype 9 carrying the human NGLY1 gene to Ngly1-deficient model rats aged 3 or 5 to 7 weeks through intracerebroventricular injection significantly improved their motor function defects [14–16]. Considering the importance of the therapeutic time window for gene therapies, early intervention may be crucial to alleviate the various symptoms caused by the dysfunction of the central nervous system in this disease. Therefore, there exists an urgent need for methods to enable the early diagnosis of NGLY1 deficiency by measuring endogenous NGLY1 activity in specimens from potential disease candidates.
A method for measuring endogenous NGLY1 activity using 5-carboxyfluorescein-labeled glycosylated cyclo-heptapeptide (5FAM-GCP) has been established previously [3,17,18]. This approach enables detecting endogenous PNGase/NGLY1 activities from various enzyme sources without the proteolytic degradation of reaction products during incubation with crude enzyme preparations. However, it requires HPLC for the separation and detection of products, which is often unavailable in standard clinical laboratories. Hence, it is crucial to develop a facile, sensitive probe for enzyme assay similar to MM3D, a fluorescence and quencher-based FRET probe designed for detecting ENGase activity [19]. We recently developed a novel FRET-based GCP probe (fGCP) consisting of a glycan modified with a fluorophore-labeled bisected-GlcNAc [aminomethylcoumarin acetate-labeled GlcNAc (AMCA-GlcNAc)] and a cyclo-heptapeptide modified with a quencher, 4-((4-(dimethylamino)phenyl)azo)benzoic acid (Dabcyl) (Figure 1) [20]. This method allows the detection of endogenous NGLY1 activity in various enzyme sources via fluorescence on multiarray plates. Our novel assay method could provide a reliable diagnostic tool and valuable insights into the regulation of PNGase/NGLY1 activities in various organisms.
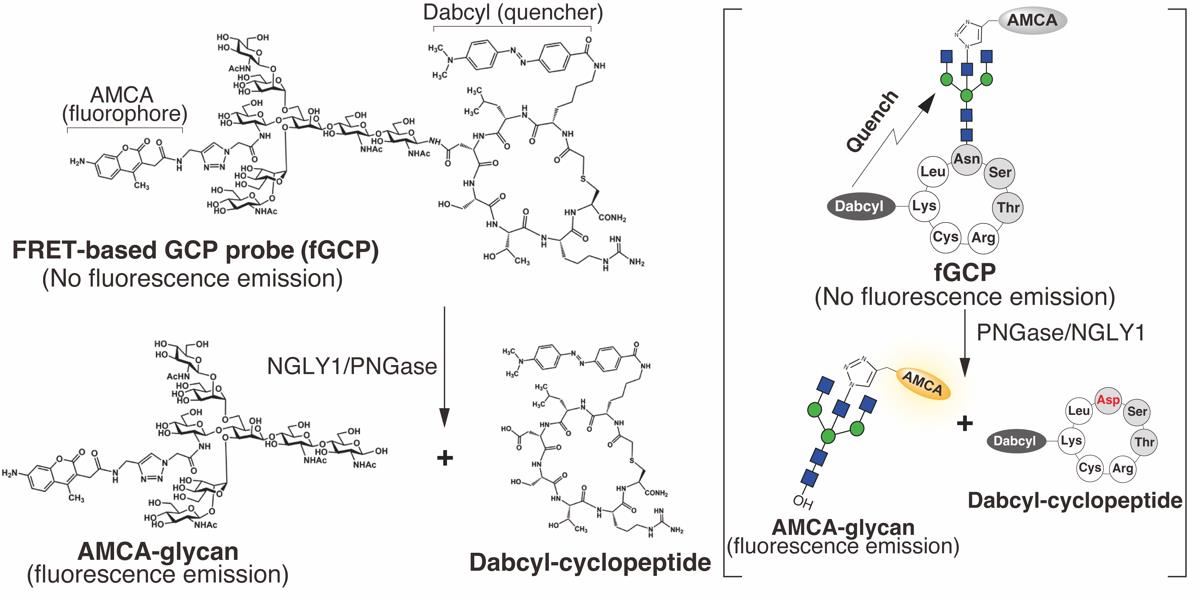
Figure 1. Structure of 5-carboxyfluorescein- and dabcyl-labeled glycosylated cyclo-heptapeptide (fGCP) and deglycosylation reaction catalyzed by PNGase [20]. The bisected-GlcNAc and lysine residues on the glycosylated cyclo-heptapeptide were labeled with a fluorophore, aminomethylcoumarin acetate (AMCA), and a quencher, 4-((4-(dimethylamino)phenyl)azo)benzoic acid (Dabcyl), respectively. The right bracket illustrates a schematic of fGCP and deglycosylation reaction catalyzed by PNGase/NGLY1.
Materials and reagents
Biological materials
1. Rat brain tissues (a rat outbred strain, Sprague-Dawley)
2. Cell lines (e.g., HeLa and HEK293 cells)
3. Fibroblast derived from healthy subjects and NGLY1-deficiency patients (available from Coriell.org)
Reagents
1. 5-Carboxyfluorescein- and dabcyl-labeled glycosylated cyclo-heptapeptide (fGCP, Mw: 2924.9) (GlyTech, Inc.)
2. Sucrose (FUJIFILM Wako Chemicals, catalog number: 196-00015)
3. EDTA (FUJIFILM Wako Chemicals, catalog number: 345-01865)
4. Trizma base (Sigma-Aldrich, catalog number: T1503)
5. NP-40 (IGEPAL® CA-630) (MPBIO, catalog number: 198596)
6. Hydrochloric acid (HCl) (FUJIFILM Wako Chemicals, catalog number: 080-01066)
7. cOmplete EDTA-free protease inhibitor cocktail (Merck-Millipore, catalog number: 11836170001)
8. Pefabloc SC (Merck-Millipore, catalog number: 11429868001)
9. Rabeprazole sodium salt (Tokyo Chemical Industry Co. Ltd., catalog number: R0115)
10. Dithiothreitol (DTT) (FUJIFILM Wako Chemicals, catalog number: M02712)
11. Powermasher II (Nippi-Inc., catalog number: 891-300)
12. Biomasher II (1.5 mL tube) (Nippi-Inc., catalog number: 320-103)
Solutions
1. 10× NGLY1 buffer (see Recipes)
2. Lysis buffer for animal tissues (see Recipes)
3. Lysis buffer for cultured cells (see Recipes)
4. 1 mM fGCP stock solution (see Recipes)
5. 100 μM fGCP working solution (see Recipes)
Recipes
1. 10× NGLY1 buffer
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 M Tris-HCl (pH 7.5) | 50 mM | 5 mL |
| Sucrose | 10 mM | 342 mg |
| 500 mM EDTA (pH 8.0) | 5 mM | 1 mL |
| Total | n/a | 100 mL |
Store at room temperature. This buffer remains stable at room temperature for at least one year.
2. Lysis buffer for animal tissues
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× NGLY1 buffer | 1× | 100 μL |
| 100 mM DTT | 1 mM | 10 μL |
| 100 mM Pefabloc SC | 1 mM | 10 μL |
| 50× protease inhibitor cocktail | 1× | 20 μL |
| 5 mM Rabeprazole | 50 μM | 10 μL |
| Distilled water | n/a | 850 μL |
| Total | n/a | 1 mL |
40 μL of lysis buffer is used for one reaction. The reagent should be prepared immediately before use.
3. Lysis buffer for cultured cells
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 10× NGLY1 buffer | 1× | 100 μL |
| 10% (v/v) NP-40 | 0.5% (v/v) | 50 μL |
| 100 mM DTT | 1 mM | 10 μL |
| 100 mM Pefabloc SC | 1 mM | 10 μL |
| 50× protease inhibitor cocktail | 1× | 20 μL |
| 5 mM Rabeprazole | 50 μM | 10 μL |
| Distilled water | n/a | 800 μL |
| Total | n/a | 1 mL |
40 μL of the buffer is used for each reaction. The reagent should be prepared immediately before use.
4. 1 mM fGCP stock solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| fGCP | 1 mM | 1 mg |
| Distilled water | n/a | 342 μL |
Store at -20 °C. This stock remains stable at -20 °C for at least 1–2 years.
5. 100 μM fGCP working solution
| Reagent | Final concentration | Quantity or Volume |
|---|---|---|
| 1 mM fGCP | 100 μM | 1 μL |
| Lysis buffer | n/a | 9 μL |
| Total | n/a | 10 μL |
10 μL of the solution is used for each reaction.
Laboratory supplies
1. 96-well black polystyrene microplate (clear flat bottom) (Corning, catalog number: CLS3603)
Equipment
1. Refrigerated microcentrifuge
2. Sonicator (TOMY, model: UR-21P)
3. Varioskan LUX multimode microplate reader (Thermo, model: VL0000D0)
Procedure
文章信息
稿件历史记录
提交日期: Aug 25, 2024
接收日期: Nov 6, 2024
在线发布日期: Nov 20, 2024
出版日期: Jan 5, 2025
版权信息
© 2025 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Hirayama, H. and Suzuki, T. (2025). An Assay System for Plate-based Detection of Endogenous Peptide:N-glycanase/NGLY1 Activity Using A Fluorescence-based Probe. Bio-protocol 15(1): e5151. DOI: 10.21769/BioProtoc.5151.
Hirayama, H., Tachida, Y., Fujinawa, R., Matsuda, Y., Murase, T., Nishiuchi, Y. and Suzuki, T. (2024). Development of a fluorescence and quencher-based FRET assay for detection of endogenous peptide:N-glycanase/NGLY1 activity.J Biol Chem. 300(4): 107121.
分类
生物化学 > 蛋白质 > 荧光
生物化学 > 蛋白质 > 定量
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link