- EN - English
- CN - 中文
Live Visualization of Calcified Bones in Zebrafish and Medaka Larvae and Juveniles Using Calcein and Alizarin Red S
利用卡尔青和苋红S实时观察斑马鱼和青鳉幼体及青年阶段的钙化骨骼
(§ Technical contact) 发布: 2024年12月20日第14卷第24期 DOI: 10.21769/BioProtoc.5142 浏览次数: 2398
评审: Alberto RissoneAmr Galal Abdelraheem IbrahimAnonymous reviewer(s)
Abstract
Zebrafish and medaka are valuable model vertebrates for genetic studies. The advent of CRISPR-Cas9 technology has greatly enhanced our capability to produce specific gene mutants in zebrafish and medaka. Analyzing the phenotypes of these mutants is essential for elucidating gene function, though such analyses often yield unexpected results. Consequently, providing researchers with accessible and cost-effective phenotype analysis methods is crucial. A prevalent technique for investigating calcified bone development in these species involves using transgenic fish that express fluorescent proteins labeling calcified bones; however, acquiring these fish and isolating appropriate crosses can be time-consuming. We present a comprehensive protocol for visualizing ossified bones in zebrafish and medaka larvae and juveniles using calcein and alizarin red S staining, which is both economical and efficient. This method, applicable to live specimens during the ossification of bones, avoids apparent alterations in skeletal morphology and allows for the use of different fluorescent dyes in conjunction with transgenic labeling, thus enhancing the analysis of developmental processes in calcifying bones, such as vertebrae and fin rays.
Key features
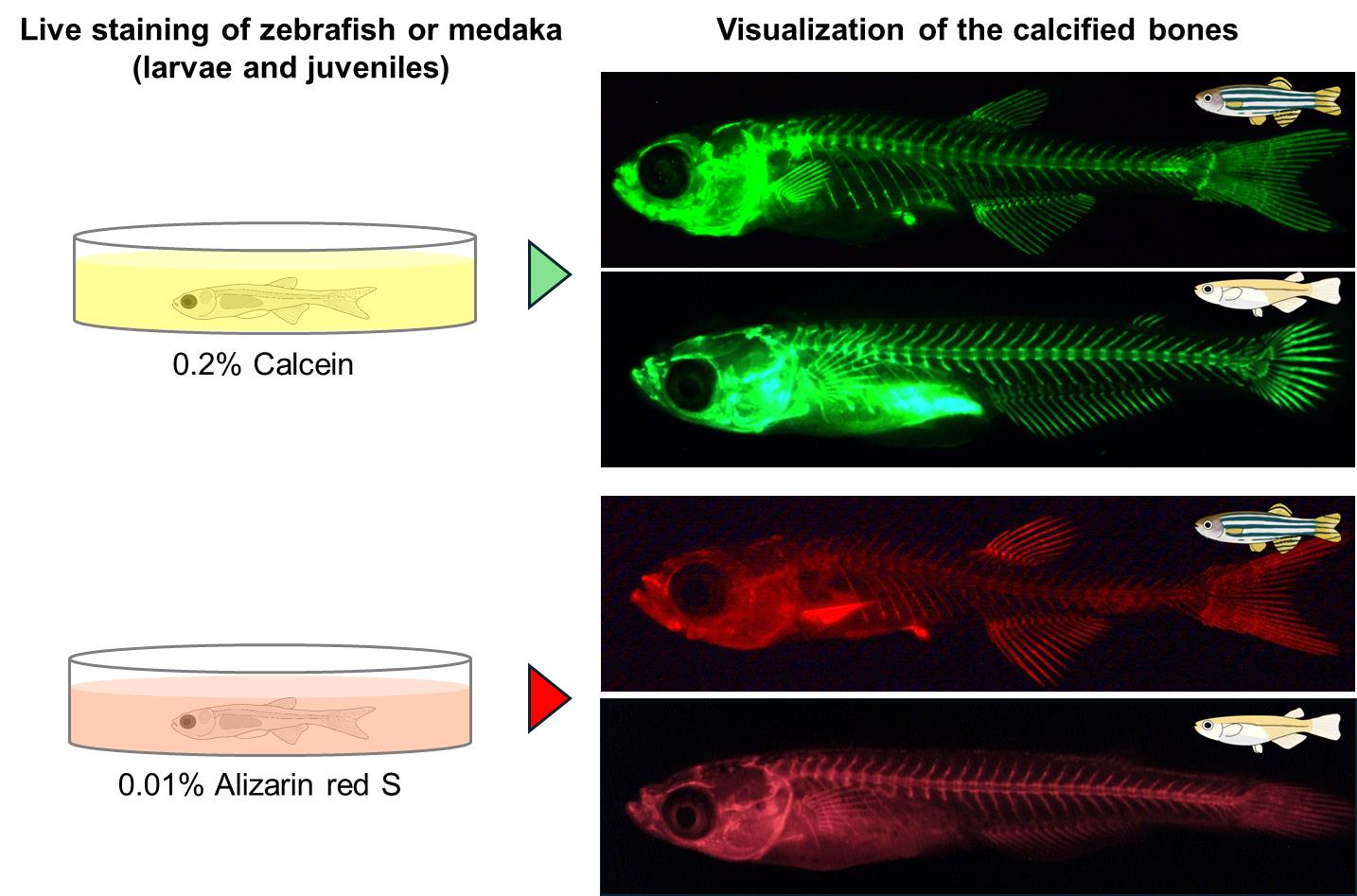
• The calcified bones of alive zebrafish and medaka larvae and juveniles can be visualized repeatedly using simple and inexpensive calcein and alizarin red S.
• No need to use transgenic fish to visualize ossified bones, allowing for rapid analysis of bone phenotypes in mutants.
• Double staining is possible in transgenic fish with reporter genes such as GFP and DsRed using alizarin red S or calcein, which exhibit different fluorescence.
• Ossification processes of bones such as vertebrae, ribs, and fin rays can be analyzed in mutants.
Keywords: ZebrafishGraphical overview
Background
Zebrafish and medaka are model vertebrates that are amenable to genetic approaches. Chemical mutagenesis has led to the isolation of hundreds of mutants in these species [1,2], contributing greatly to our knowledge of vertebrate development and other biological phenomena. The development of CRISPR-Cas9 technology has further enhanced our ability to generate mutants of specific genes in vertebrate models [3–5]. To fully understand the function of a gene, it is crucial to analyze the phenotype of the mutants. However, the generation of mutants often results in unexpected phenotypes. Therefore, it is important to provide researchers with accessible and cost-effective methods to analyze the phenotypes.
A common approach to studying the development of calcified bones in zebrafish and medaka is to use transgenic fish that specifically express fluorescent proteins labeling calcified bones [6,7]. However, it is necessary to first obtain transgenic fish, especially for researchers who do not specialize in bone research. Even if they manage to obtain them, it takes several months to analyze them because new fish have to be isolated by crossing transgenic fish with the mutant fish. Here, we describe a detailed step-by-step protocol for visualizing the ossified bones of zebrafish and medaka larvae and juveniles. This method allows the visualization of calcified bones in zebrafish and medaka by staining with calcein and alizarin red S, which are inexpensive reagents. The staining can be performed in one or two days on live zebrafish and medaka and can be repeated multiple times, as it does not cause any noticeable abnormalities in the subsequent skeletal morphology such as vertebrae and fin rays [8,9]. In addition, calcein and alizarin red S are different fluorescent dyes, and double staining is possible by using different fluorescent dyes depending on the fluorescent protein in transgenic fish. Analysis of mutants using this method is expected to improve our understanding of various developmental processes in the calcifying bones of zebrafish and medaka, including vertebrae and fin rays.
Materials and reagents
Biological materials
Zebrafish (Danio rerio, Riken Wild-type (RW) strain, the National BioResource Project Zebrafish in Japan)
Medaka (Oryzias latipes, Hd-rR strain, the National BioResource Project Medaka in Japan)
Reagents
Calcein (Fujifilm Wako, catalog number: 340-00433)
NaOH (Fujifilm Wako, catalog number: 194-18865)
NaCl (Fujifilm Wako, catalog number: 191-01665)
KCl (Fujifilm Wako, catalog number: 160-03555)
CaCl2 (Fujifilm Wako, catalog number: 038-19735)
HEPES (Fujifilm Wako, catalog number: 342-01375)
Alizarin red S (Fujifilm Wako, catalog number: 011-01192)
KOH (Fujifilm Wako, catalog number: 168-21815)
Tricaine (Fujifilm Wako, catalog number: 051-06571)
2-Phenoxyethanol (Fujifilm Wako, catalog number: 163-12075)
Methylcellulose 1500 cP (Fujifilm Wako, catalog number: 139-02145)
Solutions
2.0% calcein stock solution (see Recipes)
0.2% calcein staining solution (see Recipes)
1× Ringer’s solution (see Recipes)
1/3× Ringer’s solution (see Recipes)
0.1% alizarin red S stock solution (see Recipes)
0.01% alizarin red S staining solution (see Recipes)
Tricaine (MS-222) anesthetizing stock solution for zebrafish (see Recipes)
2.0% methylcellulose solution (see Recipes)
Recipes
2.0% calcein stock solution
Reagent Final concentration Amount Calcein 2.0% 1 g 0.5 N NaOH solution Adjust to pH 7.0 1/3 Ringer’s solution (Recipe 4) n/a Add up to 50 mL Total n/a 50 mL The prepared 2.0% calcein stock solution should be aliquoted into small tubes and stored at -20 °C in the dark. Instead of 1/3 Ringer’s solution, E3 or similar buffers can be used. Prepare a 0.5 N NaOH solution by dissolving 1.0 g of NaOH in 50 mL of distilled water.
0.2% calcein staining solution
Reagent Final concentration Amount 2.0% calcein stock solution (Recipe 1) 0.2% 300 μL 1/3 Ringer’s solution (Recipe 4) n/a 2.7 mL Total n/a 3.0 mL 0.2% calcein staining solution should be prepared prior to staining. The example shown above is for a 35 mm dish; the volume to be added is 3.0 mL. If more dishes are used, the volume can be increased. Instead of 1/3 Ringer’s solution, E3 or similar buffers can be used.
1× Ringer’s solution
Reagent Final concentration Amount NaCl 116 mM 6.78 g KCl 2.9 mM 0.216 g CaCl2·2H2O 1.8 mM 0.265 g HEPES 5.0 mM 1.192 g 0.5 N NaOH solution Adjust to pH 7.0 DW n/a Add up to 1,000 mL Total n/a 1,000 mL 1× Ringer’s solution is stable at room temperature for years.
1/3× Ringer’s solution
Reagent Final concentration Amount 1× Ringer’s solution (Recipe 3) 1/3× 333 mL DW n/a Add up to 1,000 mL Total n/a 1,000 mL 1/3× Ringer’s solution is stable at room temperature for years.
0.1% alizarin red S stock solution
Reagent Final concentration Amount Alizarin red S 0.1% 0.05 g 1 N KOH solution Adjust to pH 7.4 1/3 Ringer’s solution (Recipe 4) n/a Add up to 50 mL Total n/a 50 mL 0.1% alizarin red S stock solution should be stored at room temperature in the dark. Instead of 1/3 Ringer’s solution, E3 or similar buffers can be used. Prepare a 1 N KOH solution by dissolving 2.0 g of KOH in 50 mL of distilled water.
0.01% alizarin red S staining solution
Reagent Final concentration Amount 0.1% alizarin red S stock solution (Recipe 5) 0.01% 300 μL 1/3 Ringer’s solution (Recipe 4), or DW n/a 2.7 mL Total n/a 3.0 mL 0.01% alizarin red S staining solution should be prepared prior to staining. The example shown above is for a 35 mm dish; the volume to be added is 3.0 mL. If more dishes are used, the volume can be increased. Instead of 1/3 Ringer’s solution, E3 or similar buffers can be used.
Tricaine (MS-222) anesthetizing stock solution for zebrafish
Reagent Final concentration Amount Tricaine 2.0% 1 g DW Add up to 50 mL Total n/a 50 mL The prepared tricaine anesthetizing stock solution should be divided into small tubes and stored at -20 °C in the dark.
2.0% Methylcellulose solution
Reagent Final concentration Amount Methylcellulose 1500 cP 2.0% 1 g DW n/a Add up to 50 mL Total n/a 50 mL To completely dissolve the methylcellulose in DW, shake vigorously for several days. When photographing, the fish is placed in this viscous solution to orient the fish in the desired position. It is possible to adjust the solution to a percentage of 2%–3% methylcellulose depending on personal preference.
Laboratory supplies
Non-treated dish, 35 mm (IWAKI, catalog number: 1000-035)
Glass-base dish, 35 mm (IWAKI, catalog number: 3970-035)
Transfer pipette, 3 mL (BD Falcon, catalog number: 357575)
Equipment
Fluorescent stereomicroscope (Leica, model: M205 FA)
Digital camera (Leica, model: DFC350 FX)
Software and datasets
ImageJ (v. 1.53e, September 2020, free to use, https://imagej.net/ij/)
Procedure
文章信息
稿件历史记录
提交日期: Aug 14, 2024
接收日期: Oct 15, 2024
在线发布日期: Nov 4, 2024
出版日期: Dec 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Koita, R., Oikawa, S., Tani, T., Matsuda, M. and Kawamura, A. (2024). Live Visualization of Calcified Bones in Zebrafish and Medaka Larvae and Juveniles Using Calcein and Alizarin Red S. Bio-protocol 14(24): e5142. DOI: 10.21769/BioProtoc.5142.
分类
发育生物学 > 细胞生长和命运决定 > 分化
生物科学 > 生物技术 > CRISPR/Cas9
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













