- EN - English
- CN - 中文
Application of a Dual Optogenetic Silencing-Activation Protocol to Map Motor Neurons Driving Rolling Escape Behavior in Drosophila Larvae
双光遗传学抑制-激活技术在果蝇幼虫滚动逃逸行为驱动运动神经元映射中的应用
发布: 2024年12月05日第14卷第23期 DOI: 10.21769/BioProtoc.5131 浏览次数: 1596
评审: Ivan Sanchez DiazRupkatha BanerjeeAnonymous reviewer(s)
Abstract
Drosophila larvae exhibit rolling motor behavior as an escape response to avoid predators and painful stimuli. We introduce an accessible method for applying optogenetics to study the motor circuits driving rolling behavior. For this, we simultaneously implement the Gal4-UAS and LexA-Aop binary systems to express two distinct optogenetic channels, GtACR and Chrimson, in motor neuron (MN) subsets and rolling command neurons (Goro), respectively. Upon exposure to white LED light, Chrimson permits the influx of positive ions into Goro neurons, leading to depolarization, whereas GtACR mediates chloride influx into MNs, resulting in hyperpolarization. This method allows researchers to selectively activate certain neurons while simultaneously inhibiting others within a circuit of interest, offering a unique advantage over current optogenetic approaches, which often utilize a single type of optogenetic actuator. Here, we provide a detailed protocol for the dual silencing-activation approach using GtACR and Chrimson optogenetic channels and present a robust methodological framework for investigating the neuromuscular basis of rolling in larvae. Our cost-effective and scalable approach utilizes readily accessible equipment and can be applied to study other locomotor behaviors in Drosophila larvae, thereby enhancing our understanding of the neural circuit mechanisms underlying sensorimotor transformation.
Key features
• Enables real-time manipulation of neural activity, providing insights into the immediate effects of neuronal activation and silencing on larval behavior.
• The protocol is adaptable to different experimental setups, allowing researchers to extend its application to other sensory modalities or behavioral assays.
• Offers a standardized approach to studying nociceptive behaviors.
Keywords: Drosophila (果蝇)Graphical overview
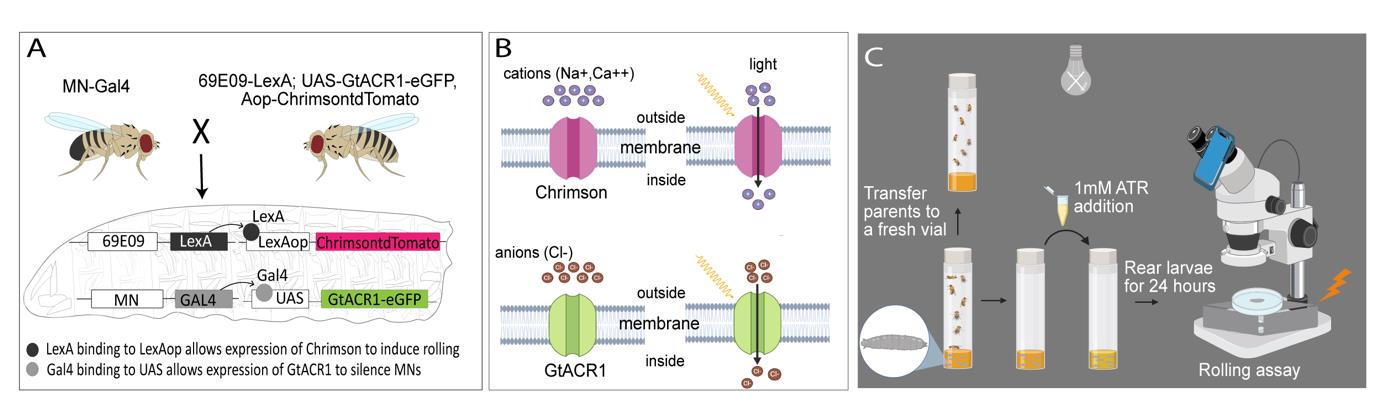
Experimental setup and procedure for the rolling assay. A) 69E06-LexA; Aop-Chrimson::tdTomato, UAS- GtACR1-eGFP female virgins are crossed with MN-Gal4 male flies, resulting in larval offspring carrying all four components essential for concurrent silencing and activation of neurons of interest. In these larvae, GtACR1-eGFP is selectively expressed in the motor neurons (MNs) of interest, whereas Aop-Chrimson::tdTomato is only expressed in Goro command neurons. B) Upon exposure to white LED light, Chrimson causes an influx of cations (Ca2+ and Na+) into Goro neurons, resulting in depolarization (activation) of these command neurons. In contrast, when exposed to white LED light, GtACR1 triggers an influx of chloride anions (Cl-), thereby hyperpolarizing (silencing) the MNs of interest. C) Both GtACR1 and Chrimson are sensitive to ambient light; therefore, the larvae should be raised in the dark until they are ready for experimentation.
Background
Noxious stimuli instinctively trigger escape behaviors in animals, prompting rapid motor responses to avoid harm. Understanding the neural mechanisms governing escape behaviors is essential for elucidating how animals respond to environmental threats. When subjected to harmful mechanical stimuli or heat, Drosophila larvae execute nocifensive escape behavior characterized by bending into a C-shape, followed by rolling and rapid forward crawling [1]. This behavior is triggered by the activation of class IV (cIV) dendritic arborization sensory neurons, which are polymodal nociceptor sensory neurons distributed throughout the body wall [2,3]. Rolling can also be induced optogenetically by stimulating nociceptive sensory neurons or interneurons within the central nervous system, including the Goro command neuron [4–6].
The body muscles of Drosophila larvae are co-innervated by type Is (small boutons, phasic firing) and a single type Ib motor neuron (MN) (big boutons, tonic firing). Type Ib MNs typically innervate one muscle, whereas Type Is MNs innervate multiple target muscles [7,8]. Here, we present a detailed protocol describing how we used a dual-optogenetic method to identify muscles and MN types essential for larval rolling escape behavior [9]. Current approaches to studying neural circuits often involve either optogenetic activation or inhibition of neural targets, limiting our ability to take full advantage of light-activated ion channels to explore circuits of interest. This protocol leverages the LexA-Aop system to express Chrimson, a cationic channelrhodopsin, in Goro neurons (69E06-LexA) to induce the larval rolling escape behavior. Concurrently, we utilize MN-Gal4 driven GtACR1, a light-sensitive chloride channel activated by the same white light as Chrimson, to acutely inhibit specific subsets of MN subsets. This dual optogenetic strategy enables the targeted and acute silencing of different MNs using different MN-Gal4 lines during the same temporal window of Goro activation. This setup, including a system to rapidly control light exposure, ensures consistent behavioral responses, thus enhancing the reproducibility and precision of behavioral studies in larvae. By implementing MN silencing in conjunction with rolling assays, we recently elucidated the neuromuscular basis of larval escape behavior in Drosophila [9].
This approach can be extended to investigate multifactorial motor neuron diseases, such as amyotrophic lateral sclerosis (ALS), which results from a combination of genetic and environmental factors that remain incompletely understood. The neural activation approach (69E06-LexA; LexA-Aop-Chrimson) can be integrated with reverse genetic screens to identify genes critical for the maintenance of motor circuit health and function, thereby elucidating novel genetic defects underlying motor neuron diseases, such as ALS. Optogenetic activation of the GORO neuron induces continuous rolling behavior in Drosophila larvae, which potentially leads to more rapid larval exhaustion than default forward crawling. The exhausted larvae may provide a more sensitive background to identify genes essential for neural function and maintenance compared to non-threatened larvae that perform forward crawling and have the opportunity to take brief pauses between crawling bouts to recover from subtle defects in motor circuit malfunction. To conduct these reverse genetic experiments, researchers can substitute UAS-GtACR1 with the UAS-RNAi line to knock down the candidate gene in the neurons of interest. Alternatively, 69E06-LexA; LexA-Aop-Chrimson activation could be employed in larvae carrying null mutant alleles for any candidate gene.
Materials and reagents
Biological materials (Drosophila stocks)
Commercially available Drosophila stocks
Type Is MN driver: w;;27E09-Gal4 (Bloomington Drosophila Stock Center, catalog number: 49227)
Type Ib MNs of ventral longitudinal (VL) and dorsal oblique (DO) muscles driver: w; exex-Gal4 (HB9-Gal4) (Bloomington Drosophila Stock Center, catalog number: 83004)
Lab-generated Drosophila stocks
Goro neuron driver: w; 69E06-LexA; Aop-Chrimson::tdTomato, UAS- GtACR1-eGFP [9]
Type Is and Ib MN1 driver: w; 27E09-Gal4; RRa-Gal4 [9]
Type Ib MNs of lateral transverse (LT) muscles driver: w; BH1-Gal4 [9]
Five type Ib MNs of dorsal longitudinal (DL) muscles driver: w; CQ-Gal4 [9]
Five type Ib MNs of DL muscles driver:: Unc4AD;vGlut DBD split Gal4 line [9]
Five Type Ib and Ib MN1 driver: w; CQ-Gal4; RRa-Gal4 [9]
Type Ib MNs of VL and DO muscles driver: w; vGlutAD;NKx6 DBD split-Gal4 [9]
Reagents
All trans-retinal (ATR) (Sigma-Aldrich, catalog number: R2500)
Agarose (Genesee Scientific, catalog number: 20-102GP)
Yellow cornmeal (Flystuff, catalog number: B078SZR5MS)
Drosophila Agar (Apex, catalog number: B078T16PNG)
Active dry yeast (SAF Instant, catalog number: B0049WLQ30)
Molasses (Oasis Supply, catalog number: B00M1ZYPXA)
Propionic acid (Genesee Scientific, catalog number: 20-271)
Tegosept (Genesee Scientific, catalog number: 20-259)
Ethanol (Sigma-Aldrich, catalog number: 64-17-5)
Solutions
Drosophila media (see Recipes)
10 mM ATR stock solution (see Recipes)
Recipes
Drosophila media
Reagent Quantity or Volume Cornmeal 67 g Drosophila agar 16 g Active dry yeast 27 g Molasses 67 mL Propionic acid 5 mL Tegosept 17 mL Distilled water 1,100 mL Begin by mixing cornmeal, agar, active dry yeast, molasses, and water in a large cooking vessel.
Place the vessel in a medium-to-high-heat setting and bring the fly food mixture to a boil.
Stir the mixture every 5–10 min to ensure even mixing.
Once boiling, reduce the heat slightly and let the mixture simmer gently for 30 min.
After 30 min of simmering, turn off the heat and allow the cooking vessel to cool until it is warm to the touch.
Carefully add the measured quantity of propionic acid and Tegosept to the warm mixture.
Transfer the well-mixed food into the Droso-Filler and pour the fly food into polypropylene vials.
Note: Any standard fly food will be suitable for this experiment; it does not need to be restricted to our recipe.
10 mM ATR stock solution
Reagent Quantity or Volume ATR 1 mg Ethanol 0.35 mL Dissolve 1 mg of ATR in 0.35 mL of ethanol to create a 10 mM stock solution.
Store the stock solution in the dark at -20 °C.
Laboratory suppliesPetri dish 10 cm Ø (e.g., Sigma-Aldrich, catalog number: CLS430167)
Spatula (Genesee Scientific, catalog number: 93-131)
Wash bottle containing distilled water (e.g., Depepe, catalog number: B07DB1HCKP)
Paintbrush (Soucolor, catalog number: B07YDDF26Y)
Wide tip forceps (DR instruments, catalog number: B008RBLO8Q)
Cellulose acetate fly plugs (VWR, catalog number: 89168-886)
Polypropylene vials (VWR, catalog number: 75813-144)
Droso-Filler (Genesee Scientific, catalog number: 59-168)
Equipment
External light source 18 W LED (remote control included) (Oeegoo, catalog number: B086JP5Y91)
Extension cord (Husky, catalog number: HD#342-576)
Microscope lens smartphone adapter (Celticbird, catalog number: B0BXWPL7H4)
Stereomicroscope (ZEISS, model: Stemi-305)
Stereomicroscope (ZEISS, model: Stemi-508)
Smartphone camera operating at 30 frames per second (fps) or more.
Note: Any smartphone brand (iPhone, Samsung, or Google Pixel) offering a high frame rate and excellent optical quality can be used. The smartphone camera lens should be aligned with the eyepiece of the microscope. A resolution of at least 12 megapixels is recommended, along with a wide-aperture lens (f/1.5 to f/1.8) to ensure optimal light capture during imaging. The default camera application on a smartphone may be used for video recording. Alternatively, camera applications such as ProCamera (iOS), Open Camera (Android), or Camera FV-5 (Android) can also be used to capture high-quality images.
Software and datasets
ImageJ (https://imagej.net/)
FFmpeg (https://ffmpeg.org/) (version 2022-08-25-git-9bf9d42d01)
Procedure
文章信息
稿件历史记录
提交日期: Jun 21, 2024
接收日期: Oct 7, 2024
在线发布日期: Oct 23, 2024
出版日期: Dec 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Sitaula, A., Huang, Y. and Zarin, A. (2024). Application of a Dual Optogenetic Silencing-Activation Protocol to Map Motor Neurons Driving Rolling Escape Behavior in Drosophila Larvae. Bio-protocol 14(23): e5131. DOI: 10.21769/BioProtoc.5131.
分类
神经科学 > 基础技术 > 光遗传学
神经科学 > 行为神经科学 > 感觉运动反应
神经科学 > 感觉和运动系统 > 动物模型
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













