- EN - English
- CN - 中文
Rapid Method for Estimating Polyhydroxybutyrate Accumulation in Bacteria Using Sodium Hypochlorite
利用次氯酸钠快速估算细菌聚羟基丁酸的积累
(*contributed equally to this work) 发布: 2024年12月05日第14卷第23期 DOI: 10.21769/BioProtoc.5130 浏览次数: 1339
评审: Andrea GramaticaDaniel Segura Segura GonzalezAlejandro Avilés-Reyes

相关实验方案
酸水解-高效液相色谱法测定集胞藻PCC 6803中聚3-羟基丁酸酯的含量
Janine Kaewbai-ngam [...] Tanakarn Monshupanee
2023年08月20日 1790 阅读
基于高效液相色谱法的史氏分枝杆菌DisA环二腺苷酸(C-di-AMP)合成酶活性研究
Avisek Mahapa [...] Dipankar Chatterji
2024年12月20日 1761 阅读
Abstract
This protocol outlines the use of the previously described sodium hypochlorite extraction method for estimating the accumulation of polyhydroxybutyrate (PHB) in bacteria. Sodium hypochlorite (NaClO) is widely used for PHB extraction as it oxidizes most components of the cells except PHB. We assessed the feasibility of using NaClO extraction for the estimation of PHB accumulation in bacterial cells (expressed as a percentage w/w). This allowed us to use a simple spectrophotometric measurement of the turbidity of the PHB extracted by NaClO as a semiquantitative estimation of PHB accumulation in the marine microorganisms Halomonas titanicae KHS3, Alteromonas sp., and Cobetia sp. However, this fast and easy protocol could be used for any bacterial species as long as some details are considered. This estimation exhibited a good correlation with the accumulation measured as dry cell weight or even with the accumulation measured by crotonic acid and HPLC quantifications. The key advantage of this protocol is how fast it allows an estimation of PHB accumulation in Halomonas, Alteromonas, and Cobetia cultures (results are available in 50 min), enabling the identification of the appropriate moment to harvest cells for further extraction, polymer characterization, and accurate quantification using more reliable and time-consuming methods. This protocol is very useful during bacterial cultivation for a quick evaluation of PHA accumulation without requiring (i) large volumes of cultures, (ii) a long time for analysis compared to dry cell weight, (iii) preparation of standard curves with sulfuric acid hydrolysis for crotonic acid quantification, or (iv) specific equipment and/or technical services for HPLC quantification.
Key features
• Fast and easy method for bacterial PHB content estimation in cultures of different marine microorganisms. It can be used in other PHB-accumulating bacteria.
• Useful to explore culture conditions to achieve maximal PHB accumulation.
• Useful to follow the kinetics of both PHB accumulation and mobilization throughout culture development.
• In cultures with high (50%–70% dry cell weight) or very low (<15%) PHB accumulation, differences are visible by the naked eye before spectrophotometric measurement.
Keywords: Polyhydroxybutyrate (聚羟基丁酸)Graphical overview
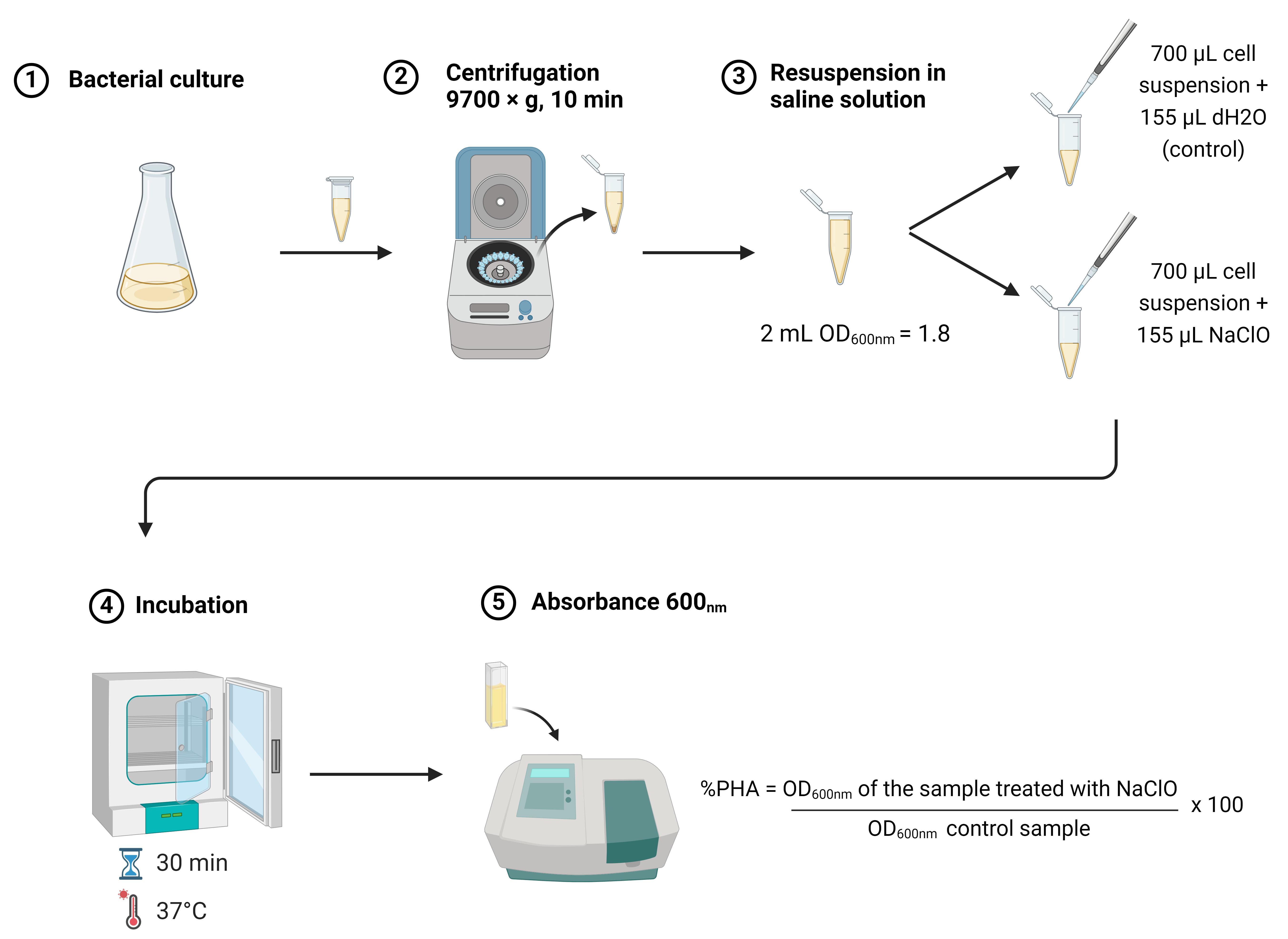
Rapid estimation of polyhydroxybutyrate content in bacteria using modified sodium hypochlorite treatment
Background
Polyhydroxyalkanoates (PHAs) are bacterial-origin polymers that share several characteristics with hydrocarbon-derived plastics. Among PHAs, polyhydroxybutyrates (PHBs) are the most common type, known for their biocompatibility and biodegradability. PHAs were first described in 1927 by Lemoigne [1] in bacteria from the genus Bacillus, and their presence in the cell was associated with the observation of Sudan Black–stained lipid inclusions. As early as 1958, Williamson and Wilkinson [2] described a method still widely used for the extraction and purification of PHAs from cells—treatment with an alkaline solution of sodium hypochlorite (NaClO). When Bacillus cells were suspended in such a solution, they were almost completely dissolved, including spores if present. Williamson and Wilkinson determined the best conditions (pH, temperature, incubation time) for the hypochlorite treatment and observed that the turbidity of the cell suspension started to decrease from the beginning of the treatment and reached a constant value when the cell lysis was complete. Moreover, the residual turbidity was proportionally higher in cell suspensions containing larger amounts of lipid inclusions as judged by microscopic observation, and they were able to show that the residual turbidity was strongly correlated with the concentration of PHA (µg/mL) remaining after the treatment. Hypochlorite treatment of Halomonas titanicae KHS3 cells that accumulated PHAs, followed by two washes with distilled water and one wash with ethanol, rendered a material that, upon NMR analysis, was found to be pure polyhydroxybutyrate [3]. PHB content could then be quantified by measuring the dry weight of hypochlorite-resistant material relative to the total dry biomass or, more precisely, through complete acid hydrolysis of the PHB material for spectrophotometric determination. However, neither of those possibilities allowed a rapid and continuous evaluation of the onset of the accumulation stage or the moment at which the cells reach a certain level of PHB content. It was then considered that the old turbidimetric measurements could be used to standardize a rapid, simple, and inexpensive method for the estimation of PHB content in growing cultures of PHB-accumulating strains. In the study conducted by Williamson and Wilkinson in 1958 [2], it was found that high cell density suspensions resulted in excessive residual turbidity, possibly due to incomplete cell lysis. As part of our protocol, we set a maximum cell density of OD600nm = 2 for the assay. By using a simple spectrophotometric measurement of the remaining turbidity after NaClO hydrolysis, our fast protocol resulted in a semiquantitative estimation of the percentage of PHB accumulation expressed as weight/weight in the marine microorganisms Halomonas titanicae KHS3, Alteromonas sp., and Cobetia sp. This estimation exhibited a reliable estimation of the accumulation, providing values that correlated to PHB weight/dry cell weight or even with the accumulation measured by crotonic acid and HPLC quantifications. Moreover, when propionic acid was added to the culture media, Halomonas titanicae KHS3 accumulated the co-polymer poly-hydroxy-butyrate-valerate (PHBV), and it was also possible to estimate PHBV accumulation using the described protocol. Therefore, although not tested here, it is highly probable that this protocol will also work for other PHAs and bacterial species.
Materials and reagents
Biological materials
Environmental strains isolated from seawater and identified as Halomonas titanicae KHS3, Alteromonas sp., and Cobetia sp.
Reagents
Dipotassium phosphate (K2HPO4) (Merck, catalog number: 105104)
Monopotassium phosphate (KH2PO4) (Fluka, catalog number: 104873)
Ammonium sulfate [(NH4)2SO4] (Merck, catalog number: 101217)
Sodium chloride (NaCl) (J.T. Baker, catalog number: 3624-19)
Magnesium sulphate heptahydrate (MgSO4·7H2O) (Anedra, catalog number: 10034-99-8)
Ferric chloride hexahydrate (FeCl3·6H2O) (Fluka, catalog number: 44944)
Sodium hypochlorite (NaClO) [5.5% (w/v) commercially available household bleach] *
Yeast extract (YE) (Oxoid, catalog number: LP0021)
Glycerol (Gly) (BioPack, catalog number: 1620.08)
Distilled water
Bacteriological agar (Oxoid, catalog number: LP0011)
*Note: Store at room temperature. Undiluted household bleach has a shelf life of six months to one year; afterward, it degrades and loses oxidative activity.
Solutions
H1 minimal medium (see Recipes)
Yeast extract 10% (YE) (see Recipes)
Glycerol 75% (Gly) (see Recipes)
Saline solution (NaCl 2%) (see Recipes)
Recipes
Note: All solutions must be autoclaved before use.
H1 minimal medium (200 mL)
Reagent Final concentration Quantity or Volume K2HPO4 11.2 g/L 2.24 g KH2PO4 4.8 g/L 0.96 g (NH4)2SO4 2 g/L 0.4 g NaCl 20 g/L 4 g MgSO4·7H2O (1 M) 1 mM 1 mL* FeCl3·6H2O (0.5% (w/v)) 1.85 µM 0.1 mL* H2O n/a 200 mL Total n/a 200 mL *Add after autoclaving.
Note: Different carbon sources are used depending on the desired culture condition: for PHB accumulation condition, 3% glycerol; for non-PHB accumulation condition, 0.25% yeast extract. To prepare plates, add 1.5% bacteriological agar to the medium. This protocol utilizes marine halotolerant bacteria, which are cultivated in H1 minimal medium. If non-halotolerant bacteria are used, appropriate media must be prepared.
Yeast extract (YE) 10% (w/v) (20 mL)
Reagent Final concentration Quantity or Volume Yeast extract 100 g/L 2 g H2O n/a 20 mL Total n/a 20 mL Glycerol (Gly) 75% (v/v) (100 mL)
Reagent Final concentration Quantity or Volume Glycerol 945 g/L 75 mL H2O n/a 25 mL Total n/a 100 mL Saline solution (NaCl 2%) (100 mL)
Reagent Final concentration Quantity or Volume NaCl 20 g/L 2 g H2O n/a 100 mL Total n/a 100 mL Note: For the cell suspension solution when using halophilic or halotolerant microorganisms. Otherwise, use a solution with the appropriate composition (saline/buffer solution similar to the growth medium).
Laboratory supplies
Microcentrifuge tube 2 mL (Henso Medical Co. Ltd., catalog number: HSN1411)
Micropipette tips 200 μL (Henso Medical Co. Ltd., catalog number: HSN1402)
Micropipette tips 1,000 μL (Henso Medical Co. Ltd., catalog number: HSN1402)
Flask 250 mL (Pyrex, catalog number: 4442-250)
Glass Petri dishes (Pyrex, catalog number: 3160-60)
Glass reagent bottle 500 mL (Duran, catalog number: 218014459)
Equipment
Micropipette P1000 (100–1,000 μL) (Gilson, model: P1000)
Micropipette P200 (20–200 μL) (Gilson, model: P200)
Glass alcohol burner (Everglass, catalog number: EVG1381)
Mini centrifuge (Eppendorf, model: MiniSpin plus)
Cell density meter (Biochrom, model: UltrospecTM 10 Classic)
Cuvettes quartz glass (Hellma, model: 6040-UV-10-531)
Orbital incubator shaker (Amerex Instruments, model: Gyromax 727)
Autoclave 13.5 L (Ficoinox, model: SL9000)
Procedure
文章信息
稿件历史记录
提交日期: Aug 13, 2024
接收日期: Oct 10, 2024
在线发布日期: Oct 22, 2024
出版日期: Dec 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Redersdorff, I. E., Rodríguez, A. N., Escobar, M., Studdert, C. A. and Herrera Seitz, M. K. (2024). Rapid Method for Estimating Polyhydroxybutyrate Accumulation in Bacteria Using Sodium Hypochlorite. Bio-protocol 14(23): e5130. DOI: 10.21769/BioProtoc.5130.
分类
微生物学 > 微生物生物化学 > 其它化合物
生物化学 > 其它化合物
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












