- EN - English
- CN - 中文
Preparation of Protein Lysates Using Biorthogonal Chemical Reporters for Click Reaction and in-Gel Fluorescence Analysis
利用生物正交化学标记制备蛋白裂解物用于点击反应及凝胶内荧光分析
发布: 2024年11月20日第14卷第22期 DOI: 10.21769/BioProtoc.5114 浏览次数: 2027
评审: Neha NandwaniDhananjay D ShindeFan Zheng

相关实验方案

一种改良的酰基-RAC方法分离视网膜棕榈酰蛋白质组,并通过LC-MS/MS进行后续检测
Sree I. Motipally [...] Saravanan Kolandaivelu
2023年04月20日 2638 阅读

高灵敏且可调控的 ATOM 荧光生物传感器:用于检测细胞中蛋白质靶点的亚细胞定位
Harsimranjit Sekhon [...] Stewart N. Loh
2025年03月20日 2270 阅读
Abstract
Bioorthogonal chemical reporters are non-native chemical handles introduced into biomolecules of living systems, typically through metabolic or protein engineering. These functionalities can undergo bioorthogonal reactions, such as copper-catalyzed alkyne-azide cycloaddition (CuAAC), with small-molecule probes, enabling the tagging and visualization of biomolecules. This approach has greatly enhanced our understanding of cellular dynamics, enzyme targeting, and protein post-translational modifications. Herein, we report a protocol for preparing protein lysates for click reaction and in-gel fluorescence analysis, exemplified using alk-16, a terminal alkyne-functionalized stearic acid analog, to investigate proteins with fatty acylation. This protocol provides methods for the fluorescent visualization of chemical reporter–labeled proteomes or individual proteins of interest (POIs).
Key features
• Metabolic incorporation of bioorthogonal chemical reporters into proteins in living cells
• Visualization of proteomes or specific proteins labeled with chemical reporters via in-gel fluorescence analysis
• Reliable, non-radioactive methods for investigating protein fatty acylation and other post-translational modifications
Keywords: Click chemistry (点击化学)Background
Bioorthogonal chemical reporters refer to non-native, non-perturbing chemical handles that can be specifically modified in living systems through highly selective reactions with exogenously delivered probes [1]. The bioorthogonal chemical reporter strategy involves incorporating unique functionalities, such as azide, alkyne, or alkene groups, into target biomolecules using the cellular biosynthetic machinery. Chemical labeling with small-molecule probes is then achieved via bioorthogonal click reactions, which are characterized by exceptional biorthogonality, biocompatibility, rapid kinetics, and high specificity in biological environments [1]. A prominent example of such click reactions is the copper-catalyzed alkyne-azide cycloaddition (CuAAC). In this reaction, an alkyne-tagged molecule reacts with an azide-tagged molecule in the presence of copper (I) to form a stable 1,4-disubstituted 1,2,3-triazole product via a [3+2] cycloaddition [2,3]. Over the past two decades, these strategies have enabled precise tracking and analysis of proteins and other biomolecules in complex biological environments, revolutionizing our understanding of biological systems. They have been applied to monitoring dynamic changes in cellular activity, profiling various cell types, states, or mutations, identifying enzyme targets, and exploring a wide range of post-translational modifications (PTMs) [4]. Herein, we present a detailed experimental protocol for the preparation of protein lysates for click reaction and in-gel fluorescence analysis, using alk-16 as a representative example. Alk-16 is a terminal alkyne-functionalized stearic acid analog that mimics endogenous long-chain fatty acids and can undergo a click reaction, allowing for the investigation of proteins with fatty acylation [5–7]. This includes the metabolic incorporation of chemical reporters into proteins in living cells, global visualization of reporter-labeled proteins in gels by selectively reacting alkynyl chemical reporter–labeled proteins in cell lysates with azido-rhodamine, and the analysis of reporter-labeled candidate proteins using immunoprecipitation, click chemistry, and fluorescence scanning (Figure 1). We aim to provide a general protocol for in-gel fluorescence analysis of proteins that are labeled by bioorthogonal chemical reporters. While we use alk-16 as an example to describe the protocol, it is also adaptable to other bioorthogonal chemical reporters, such as alkynyl-functionalized YnLac [8] and HMGAMyne [9] to detect and identify the cellular lactylated and HMGylated proteins.
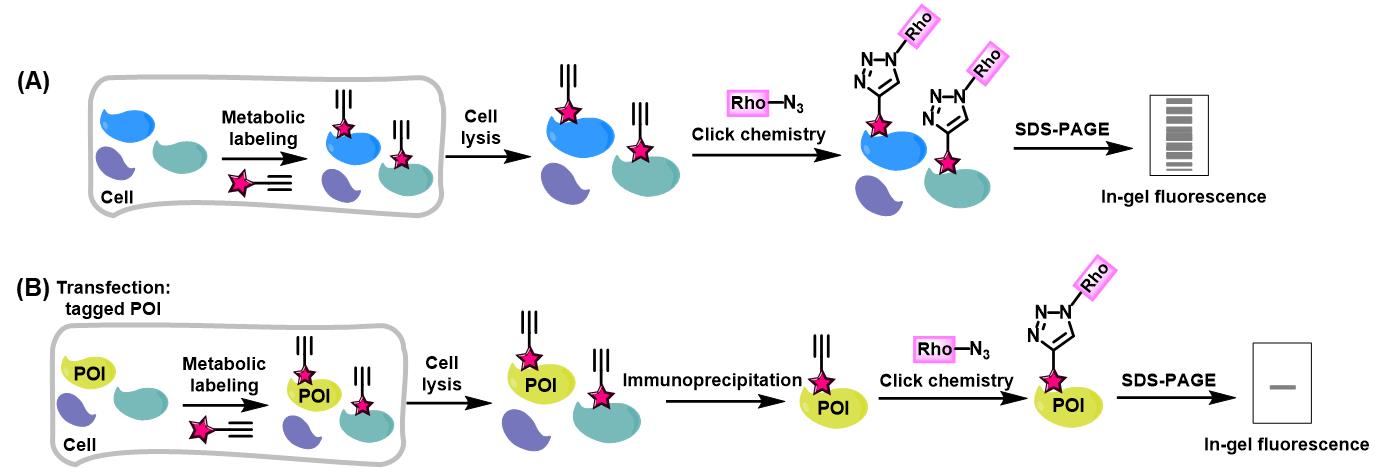
Figure 1. Workflow for in-gel fluorescence analysis of alkynyl chemical reporter–labeled proteins. (A) Schematic for in-gel fluorescence analysis of chemical reporter-labeled proteomes. (B) Schematic for in-gel fluorescence analysis of chemical reporter-labeled protein of interest (POI).
Materials and Reagents
Reagents
DMSO (Sigma-Aldrich, catalog number: D2650)
DMEM (Thermo Fisher Scientific, catalog number: 11995065) or other culture media for growing the cell type of interest
Fetal bovine serum (FBS) (Thermo Fisher Scientific, catalog number: 26140079)
Charcoal/dextran-treated fetal bovine serum (Cytiva, catalog number: SH30068)
Opti-MEM (Thermo Fisher Scientific, catalog number: 31985070)
ViaFect transfection reagent (Promega, catalog number: E4981)
Benzonase (Sigma-Aldrich, catalog number: E1014)
EDTA-free protease inhibitor cocktail (Roche, catalog number: 11873580001)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S3014)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9541)
Sodium phosphate dibasic (Na2HPO4) (Sigma-Aldrich, catalog number: S3264)
Potassium phosphate monobasic (KH2PO4) (Sigma-Aldrich, catalog number: P9791)
Sodium dodecyl sulfate (SDS) (Sigma-Aldrich, catalog number: L3771)
Trizma hydrochloride (Tris-HCl) (Sigma-Aldrich, catalog number: T3253)
Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: 258148)
Triton X-100 (Sigma-Aldrich, catalog number: T8787)
Sodium deoxycholate (Sigma-Aldrich, catalog number: D6750)
Triethanolamine (TEA) (Sigma-Aldrich, catalog number: 90279)
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: 54457)
BCA assay reagents (Thermo Fisher Scientific, catalog number: A55864)
Red anti-HA affinity gel (Sigma-Aldrich, catalog number: E6779)
Red anti-FLAG M2 affinity gel (Sigma-Aldrich, catalog number: F2426)
Anti-GFP nanobody agarose beads (AlpaLifeBio, catalog number: KTSM1301)
Tert-butanol (t-BuOH) (Sigma-Aldrich, catalog number: 471712)
Methanol (Sigma-Aldrich, catalog number: 34860)
Glycerol (Sigma-Aldrich, catalog number: G5516)
Bromophenol blue (Sigma-Aldrich, catalog number: B8026)
Bond-breaker TCEP solution, neutral pH (Thermo Fisher Scientific, catalog number: 77720)
Hydroxylamine hydrochloride (NH2OH·HCl) (Sigma-Aldrich, catalog number: 55460)
Tween-20 (Sigma-Aldrich, catalog number: 655205)
Acetic acid (Sigma-Aldrich, catalog number: A6283)
Clarity western ECL substrate (Bio-Rad, catalog number: 1705060)
Alk-16 (Sigma-Aldrich, catalog number: O8382; alternatively, it can be synthesized as described in Charron et al. [5])
Azido-rhodamine (the synthetic method has been described in Charron et al. [5])
Tris(2-carboxyethyl) phosphine hydrochloride (TCEP) (Sigma-Aldrich, catalog number: C4706)
Tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA) (Sigma-Aldrich, catalog number: 678937)
Copper sulfate pentahydrate (CuSO4·5H2O) (Sigma-Aldrich, catalog number: C8027)
Tris-MOPS-SDS running buffer (GenScript, catalog number: M00138)
Coomassie Brilliant Blue staining solution (Beyotime, catalog number: P0003S)
Solutions
50 mM alk-16 (see Recipes)
PBS (1 L) (see Recipes)
RIPA buffer (see Recipes)
1% SDS lysis buffer (see Recipes)
4% SDS buffer (see Recipes)
HEPES buffer (see Recipes)
2 M NH2OH solution (see Recipes)
5 mM azido-rhodamine stock solution (see Recipes)
50 mM TCEP solution (see Recipes)
2 mM TBTA stock solution (see Recipes)
50 mM CuSO4 solution (see Recipes)
4× SDS-PAGE loading buffer (see Recipes)
Destaining buffer (see Recipes)
Recipes
50 mM alk-16
Dissolve 14.0 mg of alk-16 in 1 mL of DMSO.
PBS (1 L)
Measure and mix the following components:
8 g of NaCl (137 mM)
0.2 g of KCl (2.7 mM)
1.44 g of NaHPO (10 mM)
0.24 g of KHPO (1.8 mM)
Add ultrapure water to a final volume of 1 L.
Adjust the pH to 7.3 using HCl.
RIPA buffer (1 L)
Measure and mix the following components:
10 mL of Triton X-100 (1% v/v)
10 g of sodium deoxycholate (1% w/v)
1 g of SDS (0.1% w/v)
8.78 g of NaCl (150 mM)
50 mL of 1 M Tris-HCl pH 7.4 (50 mM)
Add ultrapure water to a final volume of 1 L.
1% SDS lysis buffer (1 L)
Measure and mix the following components:
10 g of SDS (1% w/v)
8.78 g of NaCl (150 mM)
50 mL of 1 M HEPES pH 7.4 (50 mM)
Add ultrapure water to a final volume of 1 L.
4% SDS buffer (1 L)
Measure and mix the following components:
40 g of SDS (4% w/v)
8.78 g of NaCl (150 mM)
50 mL of 1 M TEA pH 7.4 (50 mM)
Add ultrapure water to a final volume of 1 L.
HEPES buffer (1 L)
Measure and mix the following components:
8.78 g of NaCl (150 mM)
50 mL of 1 M HEPES pH 7.4 (50 mM)
Add ultrapure water to a final volume of 1 L.
2 M NH2OH solution (10 mL)
Dissolve 1.39 g of NH2OH·HCl in 8 mL of ultrapure water.
Adjust the pH to 7.4 using NaOH.
Add ultrapure water to a final volume of 10 mL.
5 mM azido-rhodamine stock solution
Dissolve 34.3 mg of azido-rhodamine in 10 mL of DMSO.
50 mM TCEP solution
Dissolve 14.3 mg of TCEP in 1 mL of ultrapure water.
2 mM TBTA stock solution
Dissolve 10.6 mg of TBTA in 10 mL of a 1:4 (v/v) mixture of DMSO and t-BuOH.
50 mM CuSO4·5H2O solution
Dissolve 12.5 mg of CuSO4·5H2O in 1 mL of ultrapure water.
4× SDS-PAGE loading buffer (100 mL)
Measure and mix the following components:
8 g of SDS (8% w/v)
20 mL of 1 M Tris-HCl pH 6.8 (200 mM)
40 mL of glycerol (40% v/v)
0.4 g of bromophenol blue (0.4% w/v)
Add ultrapure water to a final volume of 100 mL.
Destaining buffer (1 L)
Measure and mix the following components:
500 mL of methanol (50% v/v)
100 mL of acetic acid (10% v/v)
400 mL of ultrapure water (40% v/v)
Mix thoroughly to ensure the components are well combined.
60 mm TC-treated culture dish (Corning, catalog number: 430166)
6-well clear TC-treated multiple-well plate (Corning, catalog number: 3516)
Pipette tips (Rainin, catalog numbers: 30180889, 30374583, 30296781)
10 mL serological pipette (Thermo Fisher Scientific, catalog number: 170367N)
Cell scraper (Corning, catalog number: 3010)
High-binding ELISA plate (JET-Bio, catalog number: FEP100096)
1.5 mL microfuge tube (Axygen, catalog number: MCT-150-C)
2.0 mL dolphin microcentrifuge tube (Sigma-Aldrich, catalog number: Z717533)
15-well 4%–20% Bis-Tris protein gel (GenScript, catalog number: M42015C)
Protein molecular weight standards (Yeasen, catalog number: 20351ES72)
Equipment
-80 °C freezer (Thermo Fisher Scientific, model: TDE50086FV-ULTS)
-20 °C freezer (Thermo Fisher Scientific, model: ES Series)
Biological safety cabinet (Thermo Fisher Scientific, model: 1300 Series A2)
Pipette (Rainin, model: LTS Pipette)
CO2 incubator (Thermo Fisher Scientific, model: Forma Series 3 WJ)
Inverted microscope (Nikon, model: Eclipse TS2)
Barnstead GenPure Pro ultrapure water system (Thermo Fisher Scientific, model: GenPure UV/UF)
Refrigerated centrifuge (Eppendorf, model: 5424R)
ThermoMixer (Eppendorf, model: ThermoMixer F1.5)
SpeedVac concentrator (Eppendorf, model: Concentrator Plus)
pH meter (Mettler Toledo, model: FE20K)
Multi-mode microplate reader (BioTek, model: EPOCH)
Analytical balance (Mettler Toledo, model: XS64)
Vacuum aspirator system (Dragon LAB, model: SAFEVAC)
Genie 2 vortex mixer (Scientific Industries, model: G560E)
Orbital shaker (Kylin-Bell, model: TS-200)
Dry bath incubator (Blue Pard, model: TU-10)
Protein electrophoresis system (Bio-Rad, model: PowerPac-Basic)
Trans-Blot SD semi-dry electrophoretic transfer cell (Bio-Rad, model: Trans-Blot Turbo)
ChemiDoc MP imaging system (Bio-Rad, model: ChemiDoc MP)
Procedure
文章信息
稿件历史记录
提交日期: Jul 28, 2024
接收日期: Sep 18, 2024
在线发布日期: Oct 17, 2024
出版日期: Nov 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Xu, Y. and Peng, T. (2024). Preparation of Protein Lysates Using Biorthogonal Chemical Reporters for Click Reaction and in-Gel Fluorescence Analysis. Bio-protocol 14(22): e5114. DOI: 10.21769/BioProtoc.5114.
分类
生物化学 > 蛋白质 > 荧光
生物化学 > 蛋白质 > 翻译后修饰
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link









