- EN - English
- CN - 中文
Fluorescent Staining and Quantification of Starch Granules in Chloroplasts of Live Plant Cells Using Fluorescein
利用荧光素对活植物细胞叶绿体中的淀粉颗粒进行荧光染色和定量
发布: 2024年11月05日第14卷第21期 DOI: 10.21769/BioProtoc.5103 浏览次数: 2463
评审: Wenrong HeWenyang LiSriema L. Walawage
Abstract
Plants use CO2, water, and light energy to generate carbohydrates through photosynthesis. During daytime, these carbohydrates are polymerized, leading to the accumulation of starch granules in chloroplasts. The catabolites produced by the degradation of these chloroplast starch granules are used for physiological responses and plant growth. Various staining methods, such as iodine staining, have previously been used to visualize the accumulation of chloroplast starch granules; however, these staining methods cannot be used to image live cells and/or provide confocal images with non-specific signals. In this study, we developed a new imaging method for the fluorescent observation of chloroplast starch granules in living plant cells by staining with fluorescein, a widely available fluorescent dye. This simple staining method, which involves soaking a leaf disk in staining solution, shows high specificity in confocal images. Fluorescent images of the stained tissue allow the cellular starch content of living cells to be quantified with the same level of accuracy as a conventional biochemical method (amyloglucosidase/α-amylase method). Fluorescein staining thus not only enables the easy and clear observation of chloroplast starch granules but also allows for precise quantification in living cells.
Key features
• Visualizes chloroplast starch granules stained with fluorescein in living cells.
• Requires only simple specimen preparation with no reagents needed other than the staining solution.
• Fluorescein is readily available worldwide.
• Highly specific method for identifying chloroplast starch granules in confocal images.
• Enables estimation of cellular starch content using fluorescent images.
Keywords: Starch granule (淀粉颗粒)Graphical overview
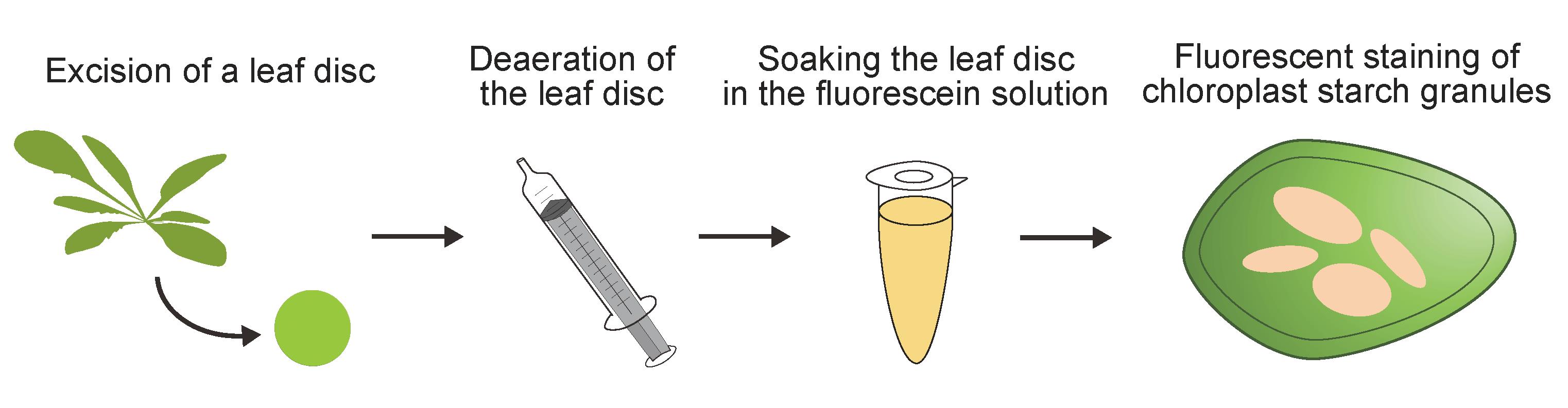
Background
Plants perform photosynthesis during the day to convert light energy into usable chemical energy. Many plant species store this energy as polysaccharides by accumulating starch granules within chloroplasts. At night, starch granules are broken down into soluble sugars, which are then distributed to sink organs for use in growth and development [1,2]. The products mobilized from starch degradation also mediate osmotic stress tolerance [3] and facilitate stomatal opening in guard cells [4]; thus, the biosynthesis and degradation of chloroplast starch granules are crucial processes in plants.
Several staining methods have been developed for observing chloroplast starch granules in leaves, enabling the quantification and investigation of their morphology. Conventional staining methods such as iodine, toluidine blue, and periodic acid Schiff staining have been widely used [5–7]; however, these staining methods involve multiple procedures and chemical fixation, preventing rapid and/or live-cell imaging. Chloroplast starch granules have also been visualized using fluorescent staining methods, including modified pseudo-Schiff propidium iodide [4,8] and safranin O staining [9], but these methods generate non-specific signals. It is therefore necessary to develop a simple staining method that enables the observation of living cells and shows good specificity for chloroplast starch granules.
To address this need, we recently established a fluorescein staining method for visualizing chloroplast starch granules fluorescently in various living plant cells [10]. Fluorescein staining only requires the submergence of leaf tissue in staining solution for 10 min. Due to its high specificity for chloroplast starch granules, fluorescent images of stained leaf tissue can be used to quantify the cellular starch content. Fluorescein staining is therefore a valuable analytical tool for understanding chloroplast starch granules in various plant species.
Materials and reagents
Biological materials
Arabidopsis thaliana accession Col-0 (Arabidopsis) (see General Note 1)
99% ethanol (Japan Alcohol Trading Co.)
Molecular sieves pack 3A (Wako, catalog number: 131-13531)
Fluorescein (Wako, catalog number: 065-00252)
Dimethyl sulfoxide (Wako, catalog number: 048-21985)
Fluorescein diacetate (FDA) (TCI, catalog number: F0240) (see General Note 2)
Fluorescein staining solution (see Recipes)
FDA staining solution (see Recipes)
Fluorescein staining solution
Reagent Final concentration Amount 10 mM fluorescein in ethanol treated with molecular sieves (100% ethanol) 10 μM 1 μL Ultrapure water n/a Up to 1,000 μL The staining solution should be prepared just before use. Molecular sieves were used as dehumidifiers for 99% ethanol following manufacturer’s instructions.
FDA staining solution
Reagent Final concentration Amount 10 mM FDA in dimethyl sulfoxide 10 μM 1 μL Ultrapure water n/a Up to 1,000 μL The staining solution should be prepared just before use.
Laboratory supplies
Hole puncher (2.0 mm in diameter) (Natsume Seisakusho Co., catalog number: KN-291-2)
Disposable 10 mL syringe and plunger (Terumo, catalog number: SS-10SZ)
Slide glass (super frost) (Matsunami Glass, catalog number: S024420)
Cover glass, 20 × 50 mm, thickness 0.16–0.19 mm (No. 1S) [Matsunami Glass, catalog number (similar product): C025501]
Microtube, 1.5 mL (WATSON, catalog number: 131-7155C)
Equipment
Confocal microscope (Leica Microsystems, model: Leica TCS SP8X)
Water-immersion lens (Leica Microsystems, model: HC PL APO 63×/1.20 W CORR CS2)
Software and datasets
LAS X (Leica Microsystems)
Fiji/ImageJ (https://imagej.net/software/fiji/downloads)
Procedure
文章信息
稿件历史记录
提交日期: Jun 21, 2024
接收日期: Sep 13, 2024
在线发布日期: Oct 12, 2024
出版日期: Nov 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ichikawa, S. and Kodama, Y. (2024). Fluorescent Staining and Quantification of Starch Granules in Chloroplasts of Live Plant Cells Using Fluorescein. Bio-protocol 14(21): e5103. DOI: 10.21769/BioProtoc.5103.
- Ichikawa, S., Sakata, M., Oba, T. and Kodama, Y. (2023). Fluorescein staining of chloroplast starch granules in living plants. Plant Physiol. 194(2): 662–672. https://doi.org/10.1093/plphys/kiad528
分类
植物科学 > 植物细胞生物学 > 细胞成像
细胞生物学 > 细胞染色 > 糖类
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













