- EN - English
- CN - 中文
Novel Cross-Species Salivary Gland-Parasympathetic Neuron Coculture System
新型跨物种唾液腺-副交感神经共培养系统
(*contributed equally to this work) 发布: 2024年11月05日第14卷第21期 DOI: 10.21769/BioProtoc.5101 浏览次数: 1964
评审: Xiaokang WuKrishna Murthy NakuluriAnonymous reviewer(s)

相关实验方案
人 iPSC 衍生神经元与少突胶质细胞共培养用于髓鞘形成的小分子筛选分析
Stefanie Elke Chie [...] Maria Consolata Miletta
2025年05月05日 3364 阅读
Abstract
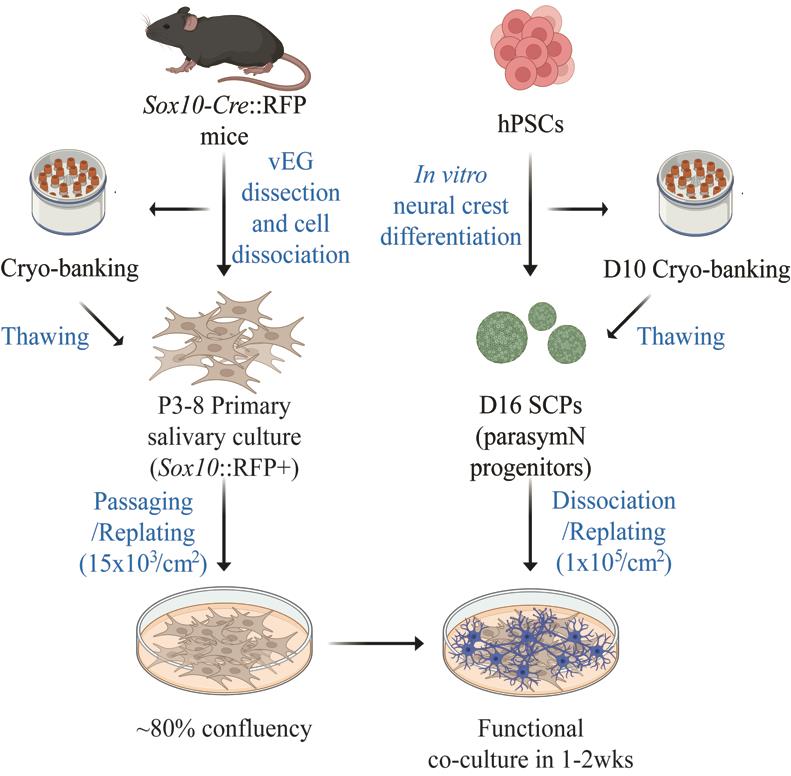
The parasympathetic nervous system is essential for salivary gland development and functionality. Parasympathetic neuron (parasymN) innervation is the main neural network that controls salivary secretion. Therefore, an exclusive model to study parasympathetic neurons and salivary gland tissue circuitry will significantly improve the understanding of the role of parasymN activation on salivary regulation. Harvesting primary rodent parasymNs is challenging due to their body-wide disbursed location. Similarly, the salivary glands are distributed in various locations around and within the oral cavity. Here, we present a coculture model system using human pluripotent stem cell (hPSC)-derived parasymNs and primary mouse von Ebner’s gland cells. We previously reported the first protocol to robustly generate human parasymNs from hPSCs through the Schwann cell precursor (SCP) lineage. The hPSC-parasymNs are functional and have been applied to model several autonomic disorders. We also used a Sox10-Cre::tdTomato (hereafter referred to as RFP) reporter mouse line, which labeled von Ebner’s glands, a type of minor salivary gland connected to the trough of circumvallate and foliate taste papillae. This labeling allowed for visualization and efficient isolation of primary tissues in young adult mice (8–10 weeks). By coculturing the two tissues, human parasymNs control mouse salivary gland cell growth and activation. Both parasymNs and primary salivary gland cells can be frozen and stocked at early stages of differentiation and isolation, making applications easier. This novel coculture model system could also be used to model and study related human diseases in the future, such as dry mouth syndrome.
Key features
• Differentiation of human parasymNs from hPSCs.
• Dissecting RFP+ mouse Ebner’s gland tissues for primary culture.
• Protocol to coculture human parasymNs with mouse primary salivary gland cells.
• Allows developmental and functional assessments of salivary regulation by the parasympathetic nervous system.
Keywords: Parasympathetic nervous system (副交感神经系统)Graphical overview
Background
The autonomic nervous system (ANS) regulates involuntary biological functions, such as blood pressure, heartbeat, and gland secretion. The ANS is subdivided into the sympathetic and parasympathetic nervous systems (SNS and PSNS). While sympathetic neurons (symNs) trigger the fight-or-flight response that increases the heartbeat, parasymNs trigger the rest-and-digest response to calm down the body, decreasing the heartbeat [1]. Clinically, the SNS usually draws more attention in autonomic dysregulation, because its dysfunction typically drives more dramatic and detrimental responses and symptoms [2,3]. However, innervation by parasymNs drives important functions, including salivation of the salivary glands [4]. Accordingly, PSNS activation induces elevated calcium (Ca2+) flux in salivary gland cells, which leads to increased saliva production [5].
Harvesting large numbers of primary parasymNs from rodents and humans for multiple assays or high-throughput screening is challenging. The hPSC technology can bridge this problem. hPSC-derived cells provide an unlimited source of any human cell type as desired. This is, however, highly dependent on good in vitro differentiation protocols. To date, there are several well-characterized symN differentiation protocols, including ours [6–11]. In contrast, only two parasymN protocols have been reported so far, and only our protocol generates functional hPSC-parasymNs that are derived via the proper developmental process, i.e., differentiation from neural crest cell (NCC)-derived SCPs [12]. In this protocol, the NCC fate was first induced from hPSCs. This was followed by SCP differentiation in 3D spheroid culture. Finally, SCP spheroids were replated to generate parasymNs [12]. To make research applications easier and secure reproducibility and efficiency, we showed that the protocol can be reproduced using frozen NCC stocks [12]. hPSC-parasymNs express specific genetic markers, including early autonomic markers ASCL1/PHOX2B, later cholinergic markers CHAT/VACHT/CHT/CHRM2/CHRM4, and specific parasymN markers HMX2/3[12,13]. We confirmed the electrical activity of hPSC-parasymNs, and electric activity could be manipulated via nicotine, which mimics preganglionic signaling. In cocultures with cardiomyocytes, nicotine activation downregulated their beating rate [12]. Using hPSC-parasymNs, we studied ANS responsiveness to COVID-19 infection and modeled the genetic autonomic disorder familial dysautonomia [12].
Salivary secretion, important for wetting oral surfaces and maintaining taste acuity, is dependent on the activity of innervating nerves to the salivary glands. Salivary glands include three pairs of major (parotid, submandibular, and sublingual) and numerous minor salivary glands around the mouth cavity. The von Ebner’s glands are a type of minor salivary glands with the ducts open to the trench of circumvallate and foliate taste papillae. Their connection to the taste papillae makes it easy to recognize and dissect. They are innervated by both sympathetic and parasympathetic efferent nerves, yet the salivary fluid secretion (water mobilizing) is largely controlled by parasympathetic activities [14].
Here, we describe a protocol to coculture hPSC-parasymNs with RFP+ mouse primary von Ebner’s gland cells [15,16]. We show that the proliferation of von Ebner’s gland cells is promoted when parasymNs are present. Furthermore, the number of auto-fluorescent secretory granule-positive von Ebner’s gland cells is significantly increased in the cocultures, suggesting the increased salivary cell maturation due to parasymN innervation. We also measured Ca2+ flux to represent salivary production, which is increased after parasymN stimulation in the cocultures. Impaired salivation can be seen in multiple diseases, such as Parkinson’s disease, Wilson disease, Angelman syndrome, various infections, and drug abuse [4]. Our newly established model may therefore be used to study the defects and cytotoxicity in those conditions, as well as the regenerative potential of hPSC-parasymNs on damaged salivary glands in the future.
Materials and reagents
Biological materials
Human embryonic stem cell line (WiCell, WA09, female, NIH 0062)
Sox10-Cre mice (Jackson Laboratory, stock 025807)
RFP Cre reporter mice B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J (Jackson Laboratory, stock 007914)
Reagents
Geltrex (Invitrogen, catalog number: A1413202)
Poly-L-ornithine hydrobromide (PO) (Sigma, catalog number: P3655)
Mouse laminin I (LM) (R&D Systems, catalog number: 3400-010-01)
Human fibronectin (FN) (VWR/Corning, catalog number: 47743-654)
Essential 8 medium (E8) (Gibco, catalog number: A15169-01)
Essential 8 supplement (E8) (Gibco, catalog number: A15171-01)
Essential 6 medium (E6) (Gibco, catalog number: A15165-01)
Neurobasal media (Gibco, catalog number: 21103-049)
Stem cell banker (Amsbio, catalog number: 11924)
DMEM/F12 (Thermo Fisher/Life Technologies, catalog number: 11330-057)
DMSO (Thermo Fisher/Life Technologies, catalog number: BP231-100)
Fetal bovine serum (FBS) (Atlanta Biologicals, catalog number: S11150)
B27 supplement (Thermo Fisher/Life Technologies, catalog number: 12587-010)
N2 supplement (Thermo Fisher/Life Technologies, catalog number: 17502-048)
L-Glutamine (Thermo Fisher/Gibco, catalog number: 25030-081)
GlutaMAX (Gibco, catalog number: 35050061)
Antibiotic-antimycotic (Fisher, catalog number: 15 240 096)
Accutase (Innovation Cell Technologies, catalog number: AT104500)
EDTA (Sigma, catalog number: ED2SS)
Trypsin-EDTA (Gibco, catalog number: 25200056)
Phosphate-buffered saline (PBS) (Gibco, catalog number: 14190-136)
SB431542 (Tocris/R&D Systems, catalog number:1614), make 10 mM stock
BMP4 (R&D Systems, catalog number: 314-BP), make 10 µg/mL stock
CHIR99021 (R&D Systems, catalog number: 4423), make 6 mM stock
Y27632 (R&D Systems, catalog number: 1254), make 10 mM stock
FGF2 (R&D Systems, catalog number: 233-FB/CF), make 10 µg/mL stock
NRG1 (PeproTech, catalog number: 100-03), make 100 µg/mL stock
GDNF (PeproTech, catalog number: 450), make 10 µg/mL stock
BDNF (R&D Systems, catalog number: 248-BD), make 10 µg/mL stock
CNTF (R&D Systems, catalog number: 257-NT), make 100 µg/mL stock
Ascorbic acid (Sigma, catalog number: A8960), make 100 mM stock
dbcAMP (Sigma, catalog number: D0627), make 100 mM stock
Retinoic acid (Sigma, catalog number: R2625), make 1 mM stock
Collagenase A (Tribioscience, catalog number: TBS2116-01), make 2 mg/mL stock
Dispase II (Tribioscience, catalog number: TBS2117-01), make 5 mg/mL stock
DPBS without Ca+2 and Mg+2 (Thermo Fisher/Life Technologies, catalog number: 14190144)
70% ethanol
Solutions
NCC induction medium, D0, 1 (see Recipes)
NCC induction medium, D2-10 (see Recipes)
SCP differentiation medium, D10-16 (see Recipes)
ParasymN differentiation medium, D16 (see Recipes)
Salivary gland cell medium (see Recipes)
Tongue tissue separation solution (see Recipes)
Salivary gland cell dissociation solution (see Recipes)
Dissociation termination solution (see Recipes)
Recipes
NCC induction medium, D0, 1 (50 mL)
Reagent Final concentration Quantity or Volume E6 n/a 50 mL SB431542 (10 mM stock) 10 µM 50 µL BMP4 (10 µg/mL stock) 0.2–1 ng/mL 1–5 µL CHIR99021 (6 mM stock) 300 nM 2.5 µL Y27632 (10 mM stock) 10 µM 50 µL Note: Please see Troubleshooting 1 for more information.
NCC induction medium, D2-10 (100 mL)
Reagent Final concentration Quantity or Volume E6 n/a 100 mL SB431542 10 µM 100 µL CHIR99021 0.75 µM 12.53 µL SCP differentiation medium, D10-16 (100 mL)
Reagent Final concentration Quantity or Volume Neurobasal media n/a 100 mL N2 supplement 1% 1 mL B27 supplement 2% 2 mL L-glutamine 1% 1 mL CHIR99021 3 µM 50 µL FGF2 (10 µg/mL stock) 10 ng/mL 100 µL NRG1 (100 µg/mL stock) 10 ng/mL 10 µL ParasymN differentiation medium, D16 (100 mL)
Reagent Final concentration Quantity or Volume Neurobasal media n/a 100 mL N2 supplement 1% 1 mL B27 supplement 2% 2 mL GlutaMAX 1% 1 mL FBS 1% 1 mL GDNF (10 µg/mL stock) 25 ng/mL 250 µL BDNF (10 µg/mL stock) 25 ng/mL 250 µL Ascorbic acid (100 mM stock) 200 µM 200 µL CNTF (100 µg/mL stock) 25 ng/mL 10 µL dbcAMP (100 mM stock) 200 µM 200 µL Retinoic acid (1 mM stock) 0.125 µM 12.5 µL Note: Retinoic acid should be added to the medium freshly every feeding.
Salivary gland cell medium (10 mL)
Reagent Final concentration Quantity or Volume DMEM/F12 1× 9.6 mL FBS 1% 100 µL B27 1× 200 µL Antibiotic-antimycotic 1× 100 µL Tongue tissue separation solution (2 mL)
Reagent Final concentration Quantity or Volume Collagenase A (2 mg/mL stock) 1 mg/mL 1.0 mL Dispase II (5 mg/mL stock) 2.5 mg/mL 1.0 mL PBS 1× Salivary gland cell dissociation solution (3 mL)
Reagent Final concentration Quantity or Volume Trypsin-EDTA 0.25% 3 mL Dissociation termination solution (2 mL)
Reagent Final concentration Quantity or Volume DMEM/F12 1× 1800 µL FBS 10% 200 µL
Laboratory supplies
TC-treated 6-well tissue culture plate (Corning, catalog number: 3516)
TC-treated 24-well tissue culture plate (Corning, catalog number: 3526)
TC-treated 10 cm tissue culture plate (Corning, catalog number: 430167)
Ultra-low attachment plate (Corning, catalog numbers: 07 200 601 and 07 200 602)
15 mL conical tissue culture tubes (VWR/Corning, catalog number: 89039-664)
50 mL conical tissue culture tubes (VWR/Corning, catalog number: 89039-656)
Cryovial (Thermo Fisher/Life Technologies, catalog number: 375353)
Trypan blue (Corning, catalog number: MT-25-900-CI)
P2-10, 20, 200, 1000 pipettes and tips (Eppendorf)
Parafilm (ULINE)
5 mL low retention vials (Eppendorf, catalog number: 30122348)
35 mm dish (Genesee Scientific, catalog number: 32-103G)
100 mm culture dishes (Genesee Scientific, catalog number: 32-107G)
3 mL syringes (BD, catalog number: 8194938)
30-G needle (BD, catalog number: 9193532)
70 µm cell strainer (Fisher Scientific, catalog number: 352350)
35 µm cell strainer (Electron Microscopy Science, catalog number: 64750-25)
100% CO2 gas (Airgas, CD USP50)
Plastic CO2 euthanasia chamber (Length: 10 cm, wide: 7 cm, and height: 5 cm)
Equipment
Water bath (VWR)
CellDrop automated cell counter (DeNovix)
Racks
Liquid nitrogen tank (Custom Biogenic Systems)
Freezer (-20 °C)
Refrigerator (2–8 °C)
Water bath (37 °C)
Centrifuge (Eppendorf)
37 °C incubator with 5% CO2
Class II biological safety hood
Lionheart FX microscope (BioTek)
Dissecting microscope (Olympus, model: SZX16 upright fluorescent and brightfield)
Biosafety cabinet (NUAIRE, model: GellGard, Class II, Type A2)
CO2 incubator (NUAIRE)
Centrifuge (Thermo Scientific, model: LEGEND XTR)
Light microscope (EVOS XL Core)
Tank with CO2 (Airgas)
Instruments
Surgical and fine scissors (Moria, catalog number: 9601; Fine Science Tool, catalog number: 91604-09)
Surgical and fine forceps (Inox electronic, catalog number: 91150-20; Fine Science Tool, catalog number: 112900)
Spatula (Fine Science Tool, catalog number: 10360-13)
Software and datasets
Gen5 (BioTek)
Prism v10.0.3 (GraphPad, 9/25/2023)
Fiji (Image J)
Adobe Photoshop
CellSens Dimension
Procedure
文章信息
稿件历史记录
提交日期: Jul 19, 2024
接收日期: Sep 9, 2024
在线发布日期: Oct 13, 2024
出版日期: Nov 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Wu, H. F., Ishan, M., Rashid, M. M., Liu, H. X. and Zeltner, N. (2024). Novel Cross-Species Salivary Gland-Parasympathetic Neuron Coculture System. Bio-protocol 14(21): e5101. DOI: 10.21769/BioProtoc.5101.
分类
干细胞 > 多能干细胞 > 细胞分化
细胞生物学 > 细胞工程 > 组织工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link