- EN - English
- CN - 中文
Development of the Mammary Gland in Mouse: A Whole-Mount Microscopic Analysis
小鼠乳腺的发育:整体显微分析
发布: 2024年10月20日第14卷第20期 DOI: 10.21769/BioProtoc.5089 浏览次数: 2062
评审: Chiara AmbrogioEmmanuelle Berret
Abstract
The mammary gland undergoes functional, developmental, and structural changes that are essential for lactation and reproductive processes. An overview of such unique tissue can offer clearer insights into mammary development and tumorigenesis. Compared to traditional methods, mouse mammary gland whole mount is a pivotal technique that provides three-dimensional structural perspectives on gland morphology and developmental stages, offering an inexpensive and accessible approach. This protocol outlines the tissue isolation of the mouse mammary gland and provides detailed instructions for whole-mount staining and analysis. Mammary gland tissues are carefully dissected from euthanized mice and stained with Carmine Alum to highlight the ductal structures, enabling detailed visualization of the branching patterns and morphological changes. Light microscopy is used to capture a panoramic image of the stained mammary gland, enabling the quantitative analysis of terminal end buds (TEBs) and bifurcated TEBs to further investigate mammary gland remodeling. This method can provide invaluable insights, particularly in the study of mammary gland morphogenesis and tumorigenesis, underscoring its significance in both basic research and clinical applications.
Key features
• Monitor mammary gland development within 2 days using whole-mount staining
Keywords: Mouse (小鼠)Graphical overview
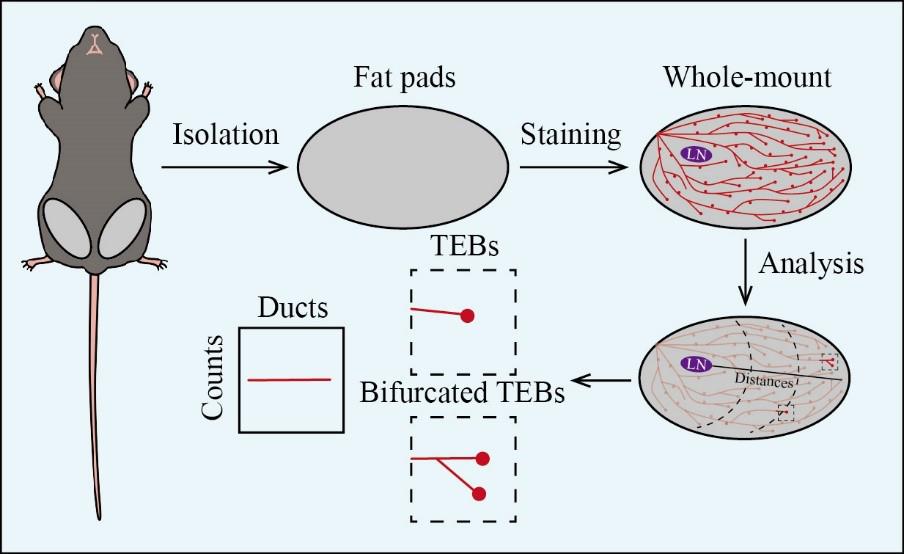
Mouse mammary gland whole-mount analysis
Background
The mammary gland undergoes dynamic developmental stages during the fetus, puberty, and adulthood stages. Developmentally, initial ductal tree formation begins around E10.5, followed by estrogen-induced formation of terminal end buds (TEBs) that drive extensive branching during puberty [1]. The most significant morphogenic event occurs during pregnancy, where lobuloalveolar structures develop at the ends of ducts for milk production [2]. Post-lactation, these structures regress, and mammary glands undergo remodeling. Therefore, the dynamic developmental stages of the mammary gland can be visually observed in three-dimensional structural morphology, which facilitates a better examination of TEBs structure. Presently, classical traditional methods include immunohistochemistry (IHC) and immunofluorescence (IF). For instance, observing the morphology of the mammary gland through hematoxylin and eosin (H&E) staining and specific epithelial cell staining via IHC allows for precise characterization [3]. IF can further label luminal and basal cells to study the functional roles of these cellular subtypes [4]. Mouse mammary gland whole mount, in contrast, is a crucial technique that provides an entire view of duct architecture and developmental processes in mammary gland development and tumorigenesis, without requiring tissue sectioning. It enables the visualization of branching morphogenesis, the formation of TEBs, and the changes in these structures. Therefore, mouse mammary gland whole-mount analysis serves as a powerful tool for advancing our understanding of mammary gland development, pathology, and oncology. This protocol typically includes several key steps: isolation of mouse mammary gland tissue, procedures for whole-mount staining, and subsequent whole-mount analysis. This method provides valuable insights into mammary gland development.
Materials and reagents
Biological materials
8-week-old female mouse
Ethanol (Sinopharm, catalog number: 10009218)
Chloroform (Sinopharm, catalog number: 10006818)
Glacial acetic acid (Sinopharm, catalog number: 10000208)
Carmine (Sigma-Aldrich, catalog number: C1022)
Aluminum potassium sulfate dodecahydrate (Solarbio, catalog number: A7520)
Hydrochloric acid (HCl) (Sinopharm, catalog number: 10011018)
Xylene (Sinopharm, catalog number: 10023418)
Permount (Fisher Scientific, catalog number: SP15-500)
Neutral balsam (Solarbio, catalog number: G8590)
Carnoy’s fixative (see Recipes)
Carmine alum (see Recipes)
Wash buffer (see Recipes)
Carnoy’s fixative (50 mL)
*Note: Prepare fixative just before use.
Reagent Final concentration Quantity or Volume Ethanol 60% (v/v) 30 mL Chloroform 30% (v/v) 15 mL Glacial acetic acid 10% (v/v) 5 mL Total (optional) n/a 50 mL* Carmine alum (100 mL)
*Note: Add approximately 90 mL of water and heat the mixture on a hot plate until it boils for 15 min. After cooling the solution, filter it using filter paper. Adjust the volume to 100 mL and store at 4 °C.
Reagent Final concentration Quantity or Volume Carmine 0.2% (w/v) 0.2 g Aluminum potassium sulfate
dodecahydrate
0.5% (w/v) 0.5 g H2O n/a see note* Total (optional) n/a 100 mL Wash buffer (100 mL)
*Note: Add water to make up to 100 mL.
Reagent Final concentration Quantity or Volume Ethanol 70% (v/v) 70 mL HCl (36%–38%) 2% (v/v) 5.64 mL H2O n/a see note* Total (optional) n/a 100 mL
Laboratory supplies
Glass slide (Citotest, catalog number: 80312)
50 mL centrifuge tubes (Corning, catalog number: 430829)
Coverslip (Citotest, catalog number: 80340)
Funnel (Citotest, catalog number: 84112)
Filter paper (Citotest, catalog number: 84501)
Volumetric cylinder (Citotest, catalog number: 84201)
Equipment
Scissors (RWD, catalog number: S12003-09)
Forceps (RWD, catalog number: F12005-10)
Autoclave (Panasonic, model: MLS-3781L-PC)
Hot plate (IKA, model: C-MAG HP 7)
Chemical fume hood (Rista lab, model: RSD-BLGTFG)
Dissecting microscope (OLYMPUS, model: SZX7)
Software and datasets
Prism v9.3 (GraphPad, 11/15/2021)
ImageJ 1.53e (NIH)
Procedure
Isolation of mouse mammary gland tissue
Euthanize an 8-week-old female mouse using CO2 inhalation.
Secure the limbs of the mice to a foam board with pushpins.
Using sterile scissors, make a skin incision on the abdomen and extend it toward the midline and hind limbs without breaching the peritoneum (Figure 1A).
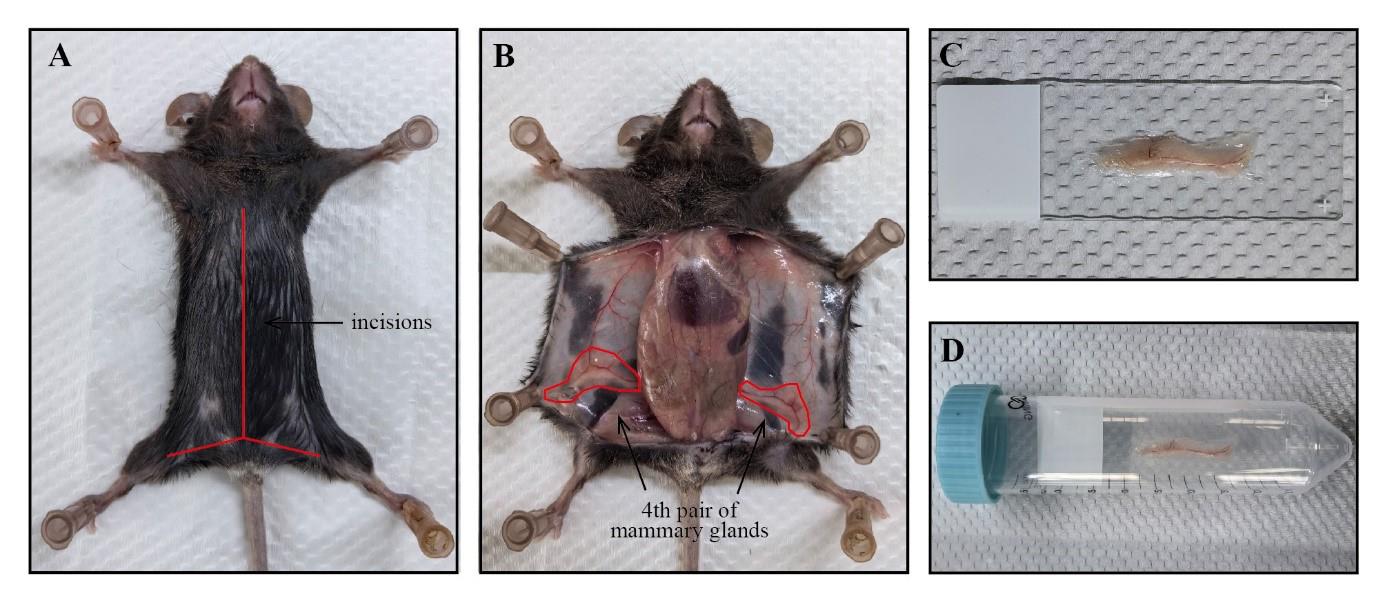
Figure 1. Isolation of mouse mammary gland tissue for whole-mount staining. A. The euthanized mouse is secured on a foam board, and its skin is incised along the midline (as indicated by the red line). B. The incised skin is pinned back to fully expose the fourth pair of mammary glands (as marked within the red circle). C. The entire mammary gland tissue is flattened on a glass slide. D. The glass slide is inserted into a centrifuge tube for staining.Securely pin the opened skin flat onto the foam board to adequately expose the fourth pair of mammary glands (Figure 1B).
Gently grasp the distal end of the entire fat pads using sterile forceps and slowly remove the mammary glands from the skin using sterile scissors. For detailed steps, refer to the video "Skin incision, removal of the mammary gland and spreading of the tissue onto glass slides" in [5].
Transfer the entire tissue onto a glass slide and gently spread the tissue using forceps (Figure 1C).
Place the glass slide at room temperature (RT) and allow the tissue edges to dry. After confirming that the tissue is securely adhered and not easily detached, transfer it into a 50 mL centrifuge tube for subsequent whole-mount staining (Figure 1D).
Whole-mount staining
Slowly add newly prepared Carnoy’s fixative to a 50 mL centrifuge tube, ensuring complete immersion of the entire mammary gland tissue in the chemical fume hood for 2–4 h or overnight at RT (Figure 2A).
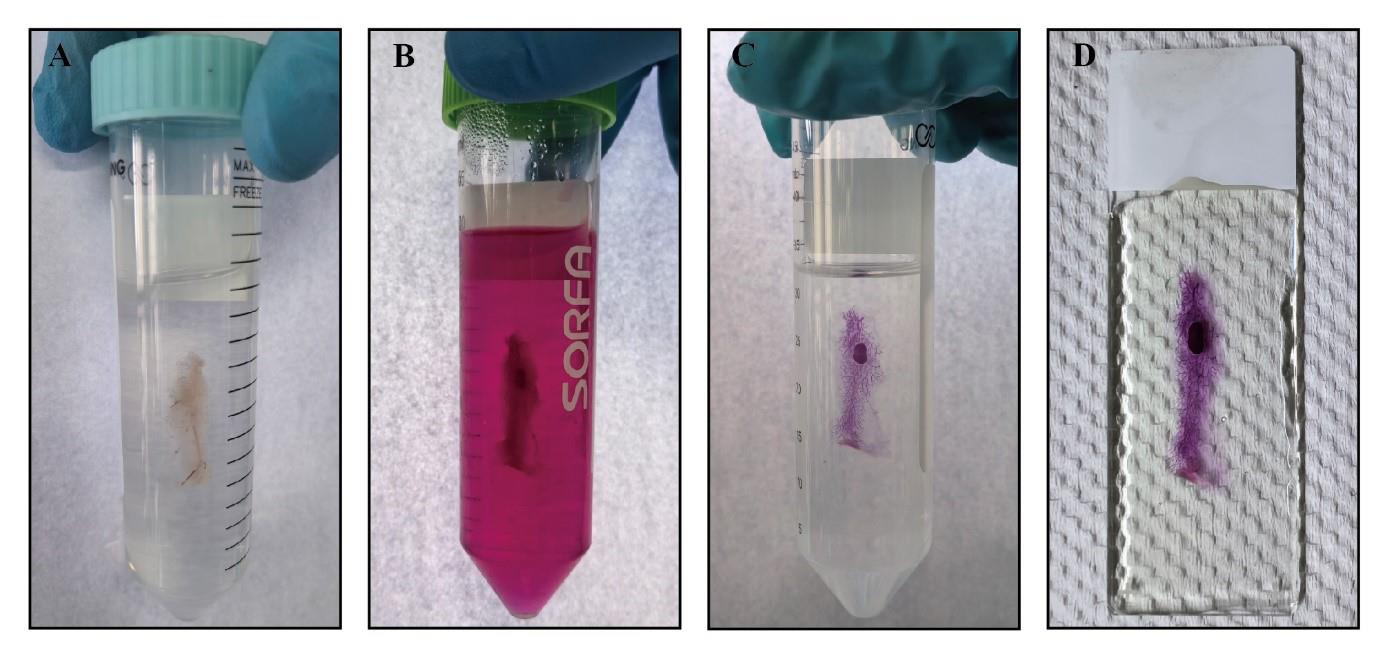
Figure 2. Process of whole-mount staining of mouse mammary gland tissue. A. The entire mammary gland tissue is fixed in Carnoy’s fixative. B. The entire mammary gland tissue is stained in Carmine Alum. C. The stained mammary gland tissue is cleared in xylene. D. The cleared mammary gland tissue is covered in neutral balsam.Transfer the glass slides to a 50 mL centrifuge tube for washing with 70% ethanol in the chemical fume hood for 15 min at RT. Mammary gland tissue processed in this step can be stored for several months.
Pass the glass slides sequentially through 50% and 30% ethanol and ddH2O in the chemical fume hood for 5 min each at RT.
Transfer the glass slides to Carmine Alum in the chemical fume hood at 4 °C overnight. Adjust staining duration based on the size and thickness of mammary gland tissue. Completed staining reveals a carmine-colored mammary epithelial tree and lymph node (Figure 2B).
Wash the glass slides with wash buffer in the chemical fume hood at RT until the solution becomes clear.
Pass the glass slides sequentially through 70%, 95%, and 100% ethanol in the chemical fume hood for 15 min each at RT.
Transfer glass slides to xylene for tissue clearing (Figure 2C) and mount them with Permount in the chemical fume hood at RT.
Apply Permount or neutral balsam to cover the entire mammary gland tissue in the chemical fume hood (Figure 2D) and store at RT.
Whole-mount analysis
Capture images of the stained entire mammary gland tissue using a dissecting microscope (Figure 3A).
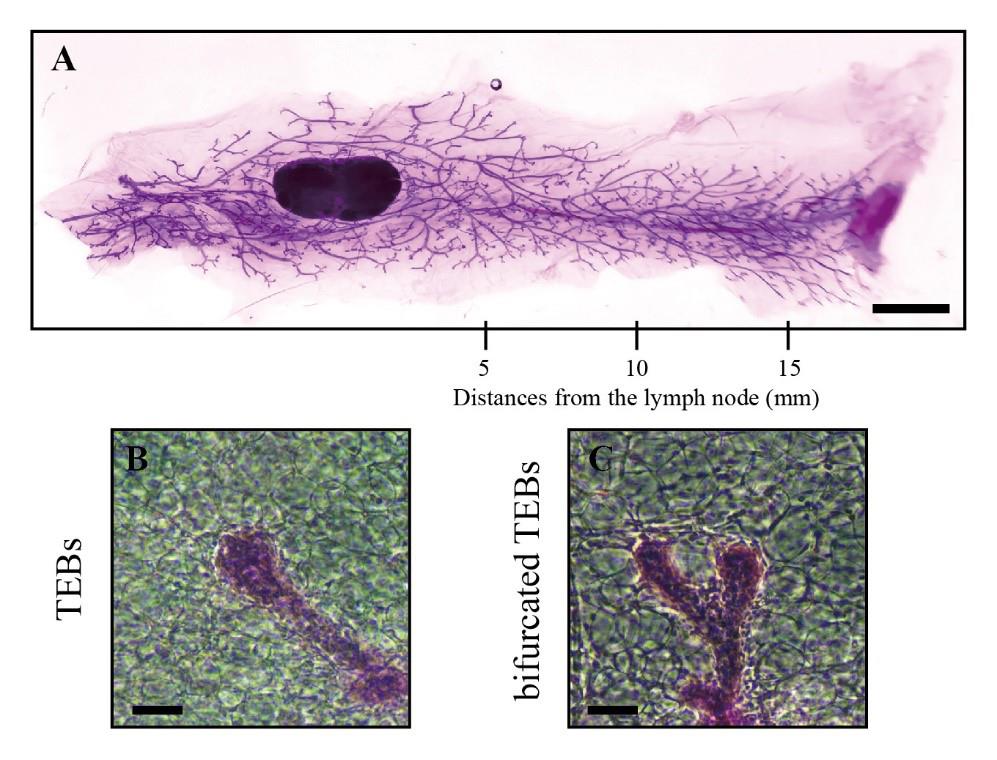
Figure 3. Information from whole-mount analysis of mouse mammary gland tissue. A. The stained entire mammary gland tissue was captured by dissecting microscopy and contains information at different distances from the lymph node (scale bars, 2.5 mm). B–C. Images of TEBs (B) and bifurcated TEBs (C) were enlarged from the panoramic image (scale bars, 100 μm).Count the number of TEBs (Figure 3B) and bifurcated TEBs (Figure 3C) directly on the images using a counter.
Count the number of ducts and acini at distances of 5, 10, and 15 mm from the lymph node along the direction of the mammary gland (Figure 3A).
Data analysis
The development of the mammary gland can be assessed directly by evaluating the mammary epithelial tree. Within this panoramic image, structures such as lymph nodes, TEBs, mammary ducts, and branches of the fourth pair of mammary glands can be distinguished (Figure 4A).
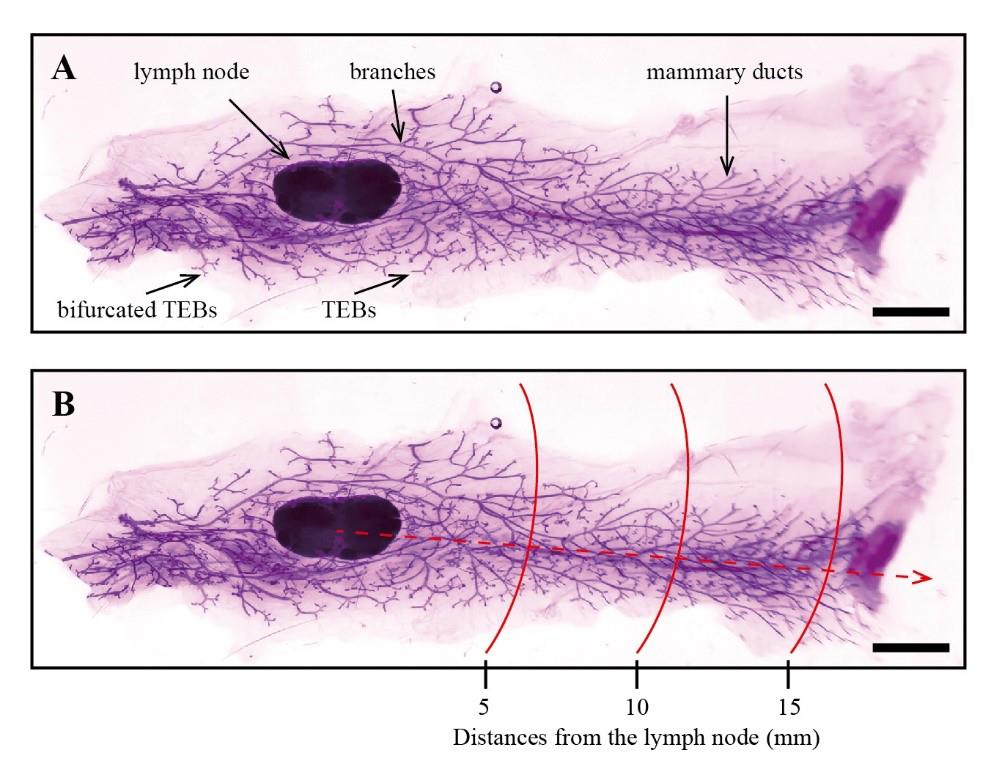
Figure 4. Examples of whole-mount analysis of mouse mammary gland tissue. A. Examples of whole-mount staining of mouse mammary gland tissue. B. Measurements of 5, 10, and 15 mm lengths (as marked within the red lines) were taken starting from the lymph node by using ImageJ (scale bars, 2.5 mm).
Prism was utilized to plot and statistically analyze the counts of TEBs and bifurcated TEBs within the entire mammary gland tissue, typically represented as "TEBs/gland (n)" and "bifurcated TEBs (n)."
Using ImageJ, the Straight tool was employed to draw scalebars length, and the scale was set using Analyze > Set Scale with the known distance input for the actual measurement of scale bars, ensuring Global was selected. Measurements of 5, 10, and 15 mm lengths were taken starting from the lymph node (Figure 4B). Prism was used to plot and statistically analyze the counts of ducts and acini within the 5, 10, and 15 mm distances from the lymph node, as well as across all distances, typically represented as "average complexity."
Validation of protocol
This protocol or parts of it has been used and validated in the following research article(s):
Wang et al. [7]. CRB3 navigates Rab11 trafficking vesicles to promote γTuRC assembly during ciliogenesis. eLife (Figure 1, panel C).
General notes and troubleshooting
General notes
Mouse mammary gland whole mount is suitable for analyzing different stages of mammary gland development in mice, including pre-pubertal (5 weeks old), pubertal (8 weeks old), pregnant, and aging (25–90 weeks of age) stages.
A 50 mL centrifuge tube can be used to stain slides of two mammary gland tissue samples placed back-to-back.
To a better demonstration of mouse mammary glands whole mount from various groups, we attempted to parallelly lay out two whole mammary gland tissues on a single slide for subsequent staining and analysis.
If Permount is not readily available, mammary gland tissue clearing can be achieved using xylene, followed by mounting with neutral balsam.
Due to the thickness of the entire mammary gland tissues, the coverslip may not always sit flat during mounting. Applying pressure can flatten the coverslip and ensure a smooth seal.
Troubleshooting
Problem 1: The stained mammary gland tissues are inadequately stained or appear faintly stained.
Possible cause: Insufficient staining time.
Solution: Increase the staining duration, potentially allowing the mammary gland tissue to stain in Carmine Alum for several days.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 82203772 and 82372931) and the Key Research and Development Program of Shaanxi Province of China (2023-YBSF-317 and 2024SF-YBXM-245). This protocol was adapted from Deng et al [6].
Competing interests
The authors declare no conflicts of interest.
Ethical considerations
This protocol involving mice was verified and approved by the Committee of Institutional Animal Care and Use of Xi'an Jiaotong University (permit number: XJTUAE2018-1801).
References
- Watson, C. J. and Khaled, W. T. (2020). Mammary development in the embryo and adult: new insights into the journey of morphogenesis and commitment. Development. 147(22): e169862. https://doi.org/10.1242/dev.169862
- Zwick, R. K., Rudolph, M. C., Shook, B. A., Holtrup, B., Roth, E., Lei, V., Van Keymeulen, A., Seewaldt, V., Kwei, S., Wysolmerski, J., et al. (2018). Adipocyte hypertrophy and lipid dynamics underlie mammary gland remodeling after lactation. Nat Commun. 9(1): 3592. https://doi.org/10.1038/s41467-018-05911-0
- Nair, S. J., Zhang, X., Chiang, H. C., Jahid, M. J., Wang, Y., Garza, P., April, C., Salathia, N., Banerjee, T., Alenazi, F. S., et al. (2016). Genetic suppression reveals DNA repair-independent antagonism between BRCA1 and COBRA1 in mammary gland development. Nat Commun. 7(1): e1038/ncomms10913. https://doi.org/10.1038/ncomms10913
- Patel, S., Sparman, N. Z. R., Arneson, D., Alvarsson, A., Santos, L. C., Duesman, S. J., Centonze, A., Hathaway, E., Ahn, I. S., Diamante, G., et al. (2023). Mammary duct luminal epithelium controls adipocyte thermogenic programme. Nature. 620(7972): 192–199. https://doi.org/10.1038/s41586-023-06361-5
- Tolg, C., Cowman, M. and Turley, E. (2018). Mouse Mammary Gland Whole Mount Preparation and Analysis. Bio Protoc. 8(13): e2915. https://doi.org/10.21769/bioprotoc.2915
- Deng, C. X. and Xu, X. (2004). Generation and Analysis of Brca1 Conditional Knockout Mice. In: Schönthal, A.H. (Eds) Checkpoint Controls and Cancer. Methods in Molecular Biology™, Humana Press. 280: 185–200. https://doi.org/10.1385/1-59259-788-2:185
- Wang, B., Liang, Z., Tan, T., Zhang, M., Jiang, Y., Shang, Y., Gao, X., Song, S., Wang, R., Chen, H., et al. (2023). CRB3 navigates Rab11 trafficking vesicles to promote γTuRC assembly during ciliogenesis. eLife. 12: e4. https://doi.org/10.7554/elife.86689.4
文章信息
稿件历史记录
提交日期: Jul 1, 2024
接收日期: Sep 1, 2024
在线发布日期: Sep 14, 2024
出版日期: Oct 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Wang, B., Xie, Y., Yang, Z., Zhang, J., Zhang, H. and Liu, P. (2024). Development of the Mammary Gland in Mouse: A Whole-Mount Microscopic Analysis. Bio-protocol 14(20): e5089. DOI: 10.21769/BioProtoc.5089.
- Wang, B., Liang, Z., Tan, T., Zhang, M., Jiang, Y., Shang, Y., Gao, X., Song, S., Wang, R., Chen, H., et al. (2023). CRB3 navigates Rab11 trafficking vesicles to promote γTuRC assembly during ciliogenesis. eLife. 12: e4. https://doi.org/10.7554/elife.86689.4
分类
发育生物学 > 形态建成 > 器官形成
细胞生物学 > 组织分析 > 组织形态学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












