- EN - English
- CN - 中文
Expansion and Precise CRISPR-Cas9 Gene Repair of Autologous T-Memory Stem Cells from Patients with T-Cell Immunodeficiencies
扩增及精确CRISPR-Cas9基因修复来自T细胞免疫缺陷患者的自体记忆性T干细胞
发布: 2024年10月20日第14卷第20期 DOI: 10.21769/BioProtoc.5085 浏览次数: 3203
评审: Alka MehraScott McCombSalma Merchant

相关实验方案

利用EpiCRISPR系统通过靶向DNA甲基化诱导Alpha TC1-6细胞产生胰岛素
Marija B. Đorđević [...] Melita S. Vidaković
2025年10月20日 1253 阅读
Abstract
The adoptive transfer of autologous, long-lived, gene-repaired T cells is a promising way to treat inherited T-cell immunodeficiencies. However, adoptive T-cell therapies require a large number of T cells to be manipulated and infused back into the patient. This poses a challenge in primary immunodeficiencies that manifest early in childhood and where only small volumes of blood samples may be available. Our protocol describes the ex vivo expansion of potentially long-lived human T memory stem cells (TSCM), starting from a limited number of peripheral blood mononuclear cells (PBMCs). Using the perforin gene as an example, we provide detailed instructions for precise gene repair of human T cells and the expansion of TSCM. The efficiency of precise gene repair can be increased by suppressing unintended non-homologous end-joining (NHEJ) events. Our protocol yields edited T-cell populations that are ready for phenotyping, genome-wide off-target analysis, and functional characterization.
Key features
• Expansion and enrichment of TSCM from PBMCs using IL-7 and IL-15.
• Phenotyping of TSCM.
• Design of “off-the-shelf” gene-repair strategies based on knock-in of a single exon or complete cDNA and design of effective guide RNAs and DNA donor templates.
• High-efficiency gene targeting using CRISPR-Cas9, recombinant adeno-associated virus serotype 6 (rAAV6), and a selective small molecule inhibitor of DNA-dependent protein kinase (DNA-PK).
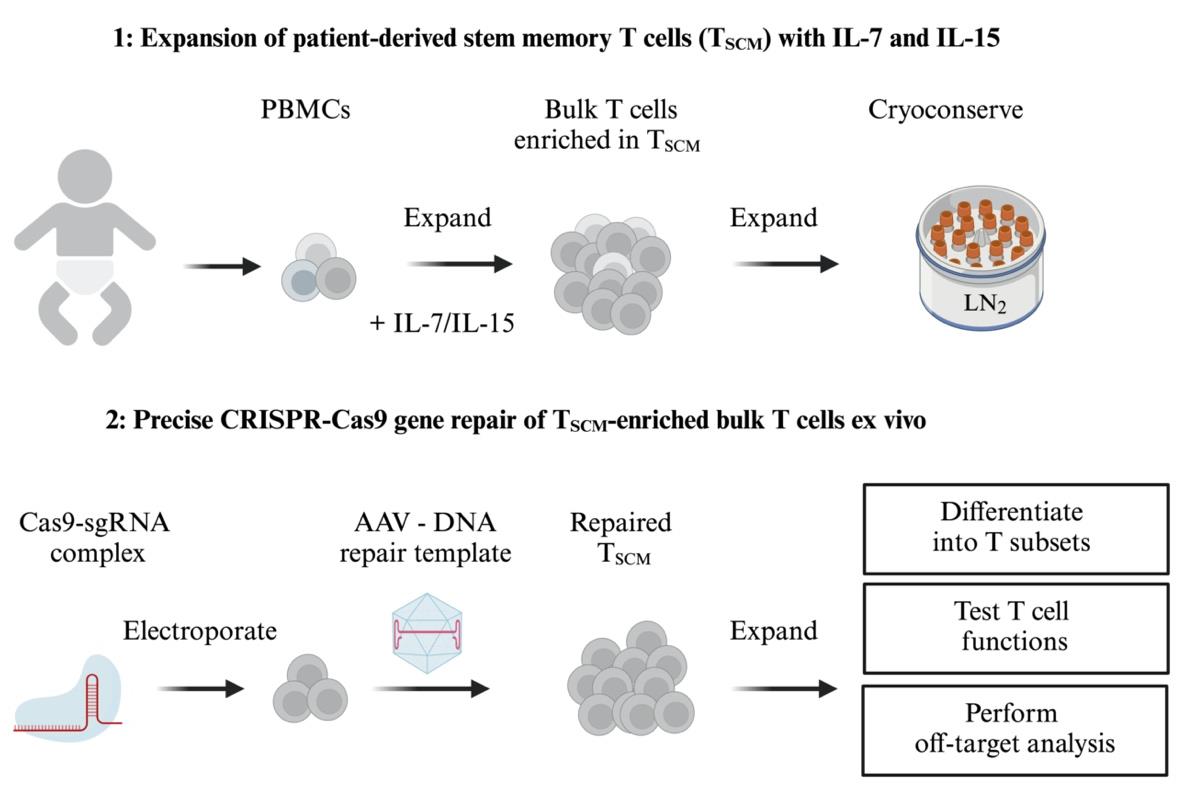
Keywords: Humans (人类)Graphical overview
Background
Familial hemophagocytic lymphohistiocytosis (FHL) is a group of inherited, potentially fatal inflammatory diseases caused by loss-of-function mutations in the NK-cell and T-cell cytotoxic machinery. Patients with FHL2 carry mutations in the perforin-1 gene (PRF1). This protocol is an extension of our previous work with primary, perforin-deficient T cells from a genetic mouse model that mimics FHL2 [1,2]. We isolated memory T cells from these mice, repaired their perforin genes, and showed that treatment with these repaired T cells was able to overcome lethal hyperinflammation [2]. The repaired T cells efficiently killed EBV-triggered malignant B cells in vivo, thereby removing the inflammatory stimulus and restoring immune homeostasis [2].
In the same study, we also showed that human T cells can be repaired in a similar way, setting the stage for translation to the clinic [2]. Ideally, T-cell therapy is performed with antigen-experienced memory T cells that are able to differentiate in vivo into different effector subsets, are long-lived, and possess self-renewing capacities [3]. T memory stem cells (TSCM) reside in CD8+ and CD4+ T-cell precursor subsets that express CD62L [4]. For the CD8 T-cell compartment, it has been shown that CD62L+ cells possess the capacity for immune reconstitution, self-renewal, and persistence in vivo [5]. A combination of recombinant IL-7 and IL-15 can be used to generate such TSCM by activating and expanding a pool of precursor T cells present in blood [6–8]. IL-7 is required for the development of these cells, IL-15 maintains their proliferation, and their gene expression signature resembles naturally occurring TSCM [6].
This protocol describes a highly efficient gene repair strategy for human primary T cells, starting from the typically limited number of peripheral blood mononuclear cells (PBMCs) that can be obtained from critically ill, immunodeficient children in a clinical setting. Ex vivo expansion and enrichment of potentially long-lived TSCM cells is followed by nucleofection of ribonucleoprotein complexes (RNPs) [9] composed of guide RNA (gRNA) and Cas9 nuclease protein to introduce site-specific DNA double-strand breaks. The T cells are then infected with recombinant adenovirus-associated virus serotype 6 (rAAV6) to deliver the DNA template for host cell–mediated homology-directed repair (HDR) [10] and are treated with a small molecule non-homologous end-joining (NHEJ) inhibitor to increase HDR efficiency [8,11]. This strategy achieves highly efficient gene repair in T cells from patients with FHL, resulting in CD8+ T-cell populations carrying more than 85% mCherry reporter knock-ins (see Suppl. Figure S2 in Li et al. [2]).
For effective gene repair, it is desirable to develop “off-the-shelf” strategies that repair as many known disease mutations as possible. We illustrate this principle using the human PRF1 locus, whose exons 2 and 3 encode a 555 amino acid polypeptide [12,13]. By replacing a mutated exon 3 with a corrected version, approximately two-thirds of ~60 known PRF1 disease mutations can be repaired [13]. By replacing exon 3 with the full-length coding region of PRF1, 20 additional disease mutations in exon 2 can be repaired. In both cases, the genetic makeup of the repaired T cells is only minimally altered, and the repaired PRF1 gene is expressed under its physiological transcription and translation control elements.
PBMC samples from FHL2 patients are difficult to obtain and therefore precious, and contain only a limited number of cells. Therefore, we also provide a protocol for cryopreservation of expanded patient TSCM cells. Our protocol does not require sorting steps for T-cell isolation and can be performed repeatedly if needed, allowing optimization of gene editing conditions, scaling up of T-cell culture, and potentially multiple rounds of patient treatment. Expanded patient T cells showed no signs of T-cell exhaustion [14–16], neither before nor after gene editing.
Materials and reagents
Biological materials
Peripheral blood mononuclear cells (PBMCs) from FHL patients and healthy donors (controls)
Reagents
OPTIONAL, see step A1 note: Addgene plasmid #64324 [pU6-(BbsI) CBh-Cas9-T2A-mCherry]
OPTIONAL, see step A1 and A2 notes: Addgene plasmid #86457 [MSCV-hU6-(BbsI)-CcdB-(BbsI)-PGK-Puro-T2A-BFP]
FuGENE® HD transfection reagent (Promega, catalog number: E2311)
Opti-MEMTM serum-reduced medium (Fisher Scientific, catalog number: 31985062)
QuickExtractTM DNA extraction solution (Lucigen, catalog number: QE0905T)
Alt-R® CRISPR-Cas9 crRNA 2 nmol [Integrated DNA Technologies (IDT)] (see Table 1 and Figure S1)
Table 1. Guide RNA (gRNA) sequences for editing the coding part of exon 3 of the PRF1 gene [gene ID 5551; Homo sapiens chromosome 10, GRCh38.14, NC_000010.11(70597348..70602741, complement]. Only the 20 target-specific nucleotides are shown in the third column (see also Figure S1). The preferred protospacer-adjacent motif (PAM) for Cas 9 is NGG, shown in bold and underlined.
Species Name Sequence (5'→3') Genome_coordinates[strand] Human gPRF1.1 GCGGGGGAGTGTGTACCACATGG Chr10:70599156:70599178[+] Human gPRF1.2 GGAGCTGGGTGGCCGCATATCGG Chr10:70599018:70599040[-] Alt-R® CRISPR-Cas9 tracrRNA 100 nmol (IDT, catalog number: 1072534) (see Figure S1)
Alt-RTMS.p. Cas9 nuclease V3 or Alt-RTMS.p. HiFi Cas9 nuclease V3 (IDT, catalog number: 1081059 or 1081061)
De novo synthesized codon-modified DNA repair template (GeneArt Gene Synthesis Services, Thermo Fisher Scientific)
pAAV-GFP Control Vector (Cell BioLabs, catalog number: VPK-400)
NEBuilder® HiFi DNA Assembly Cloning Kit (New England BioLabs, catalog number: E5520S)
One ShotTM Stbl3TM chemically competent E. coliTM (Thermo Fisher Scientific, catalog number: C737303)
ImmunoCultTM-XF T cell expansion medium (STEMCELL Technologies, catalog number: 10981)
Human recombinant IL-7 (5 ng/mL) (PeproTech, catalog number: AF-200-07)
Human recombinant IL-15 (5 ng/mL) (PeproTech, catalog number: AF-200-15)
ImmunoCultTM human CD3/CD28/CD2 T-cell activator (STEMCELL Technologies, catalog number: 10970)
CryoStor® CS10 cryopreservation medium with 10% DMSO (STEMCELL Technologies, catalog number: 07930)
Phosphate-buffered saline (PBS) pH 7.2, without calcium/magnesium (Life Technologies, catalog number: 20012-068)
Bovine serum albumin fraction V (BSA) (Carl Roth, catalog number: 8076.3)
EDTA (Ethylendiaminetetraacetic acid disodium salt) solution pH 8 (0.5 M) (Sigma-Aldrich, catalog number: 03690)
LIVE/DEADTM Fixable Near IR (876) Viability Kit (Thermo Fisher Scientific, catalog number: L34981)
Human recombinant IL-2 (25 ng/mL) (PeproTech, catalog number: 200-02)
P3 Primary Cell 4D-NucleofectorTM X kit L (Lonza, catalog number: V4XP-3012)
Dimethyl sulfoxide (DMSO) 10 mL (Sigma-Aldrich, catalog number: D2438)
M3814 (Nedisertib; DNA-PK inhibitor; CAS number: 1637542-33-6) (MedChemExpress, catalog number: HY-101570)
Ethanol absolute without additives, BioUltra 500 mL (Sigma-Aldrich, catalog number: 51976)
Phorbol-12-myristate-13-acetate (PMA) 1 mg (Sigma-Aldrich, catalog number: P8139)
Ionomycin calcium salt from S. conglobatus 1 mg (Sigma-Aldrich, catalog number: I0634)
Brefeldin A solution (1,000×) (BioLegend, catalog number: 420601)
BD Cytofix/CytopermTM Fixation/Permeabilization Kit (BD Biosciences, catalog number: 554714)
QuickExtractTM DNA extraction solution (LGC Biosearch Technologies/Lucigen, catalog number: QE09050)
PrimeSTAR® GXL DNA polymerase (TaKaRa, catalog number: R050B)
CloneJET PCR cloning kit (Thermo Fisher Scientific, catalog number: K1231)
Antibodies:
Brilliant Violet 785TM anti-human CD8, clone SKI (BioLegend, catalog number: 344740)
APC anti-human CD62L, clone DREG-56 (BioLegend, catalog number: 304810)
PE/Cyanine7 anti-human CD45RA, clone HI-100 (BioLegend, catalog number: 304126)
FITC anti-human CD95 (Fas), clone DX2 (BioLegend, catalog number: 305606)
Brilliant Violet 421TM anti-human CD279 (PD-1), clone NAT105 (BioLegend, catalog number: 367422)
PE/Dazzle TM 594 anti-human TIGIT (VSTM3), clone A15153G (BioLegend, catalog number: 372716)
PE anti-human Perforin, clone B-D48 (BioLegend, catalog number: 353304)
Optional, see step C15: PE anti-human CD366 (Tim-3), clone F38-2E2 (BioLegend, catalog number: 345006)
Optional, see step C15: Brilliant Violet 711TM anti-human CD223 (LAG-3), clone 11C3C65 (BioLegend, catalog number: 369319)
Optional, see step C15: APC/Cyanine7 anti-human CD39, clone A1 (BioLegend, catalog number: 328226)
Solutions
Bovine serum albumin (BSA) stock solution (10%) (see Recipes)
MACS buffer (see Recipes)
P3 electroporation buffer (see Recipes)
M3814 (Nedisertib) stock solution (25 mM) (see Recipes)
Phorbol myristate acetate (PMA) stock solution (50 ng/mL) (see Recipes)
Ionomycin stock solution (500 ng/mL) (see Recipes)
Recipes
Bovine serum albumin (BSA) stock solution (10%)
Layer 10 g of BSA over 100 mL of PBS in a glass beaker and let it dissolve overnight at 4 °C. Sterile-filter through a 0.2 µm PES bottle-top filter. Store at 4 °C or freeze in aliquots at -20 °C.
MACS buffer (PBS pH 7.2 containing 0.5% BSA and 2 mM EDTA)
To 500 mL of PBS pH 7.2 (without calcium and magnesium), add 25 mL of sterile 10% BSA stock solution and 2 mL of 0.5 M EDTA solution pH 8. Sterile-filter through a 0.2 µm PES bottle-top filter.
CRITICAL: LIVE/DEADTM fixable stains are only compatible with flow cytometry buffers containing less than 1% of protein.
P3 electroporation buffer
Just before use, freshly prepare 20 µL of P3 electroporation buffer by adding 3.6 µL of Supplement 1 to 16.4 µL of P3 primary cell NucleofectorTM solution (P3 Primary Cell 4D-NucleofectorTM X kit, Lonza).
M3814 (Nedisertib) stock solution (25 mM)
Dissolve 5 mg of M3814 in 415 µL of DMSO (25 mM = 5,000×). Store in 10 µL aliquots at -80 °C. Dilute the stock solution 1:100 in PBS pH 7.2 to make a 50× working stock solution (250 µM = 50×). Aliquot the working stock solution and store at -20 °C for up to 4 weeks.
Phorbol myristate acetate (PMA) stock solution (50 ng/mL)
Dissolve 1 mg of PMA in 20 mL of ethanol (50 ng/mL final concentration). Store in 10 µL aliquots at -20 °C.
CAUTION: Phorbol ester is a tumor promoter. Wear personal protective equipment including gloves.
Ionomycin stock solution (500 ng/mL)
Dissolve 1 mg of ionomycin calcium salt in 2 mL of ethanol (500 ng/mL final concentration). Store in 10 µL aliquots at -20 °C.
Laboratory supplies
Tissue culture 96-well plates, flat bottom (Sarstedt, catalog number: 83.3924)
Tissue culture 96-well plates, U bottom (Sarstedt, catalog number: 83.3926.500)
Tissue culture 48-well plates (Sarstedt, catalog number: 83.3923)
Tissue culture 24-well plates (Sarstedt, catalog number: 83.3922)
Tissue culture 12-well plates (Sarstedt, catalog number: 83.3921)
Tissue culture 6-well plates (Sarstedt, catalog number: 83.3920)
Tissue culture dish 100 × 20 mm (Sarstedt, catalog number: 83.3902)
PIPETMAN® single-channel pipettes P10, P20, P200, P1000 (Gilson)
Pipette filter tips [Sarstedt, catalog numbers: 70.3010.275 (10 µL), 70.3030.265 (20 µL), 70.3030.375 (100 µL), 70.3030.110 (200 µL), 70.3060.275 (1,000 µL)]
0.2 mL PCR tubes with domed lids, 8-well strips (neoLab, catalog number: 7-5207)
1.5 mL tubes (Eppendorf, catalog number: 0030 120.086)
2.0 mL tubes (Eppendorf, catalog number: 0030 120.094)
15 mL conical centrifugation tubes FalconTM 352097 (Thermo Fisher, catalog number: 10136120)
Mr. FrostyTM freezing container (Thermo Fisher, catalog number: 5100-0036)
2 mL CryoPure tubes (freezing vials) (Sarstedt, catalog number: 72.380.992)
Disposable PES filter units 0.2 µm, 150 mL (Thermo Scientific Nalgene, catalog number: 596-3320)
Disposable PES filter units 0.2 µm, 500 mL (Thermo Scientific Nalgene, catalog number: 595-3320)
Standard 96-well microtiter plate, U bottom (Thermo Scientific Abgene, catalog number: AB0796)
Equipment
Tissue culture incubator set at 37 °C, 5% CO2
Refrigerated centrifuge with adapters for plates and 15 mL conical tubes (e.g., Eppendorf Centrifuge 5810R)
Benchtop centrifuge for 1.5 mL/2 mL tubes (e.g., Eppendorf Centrifuge 5424)
BD LSRFortessa cell analyzer flow cytometer (BD Bioscience)
TC20TM automated cell counter (Bio-Rad, catalog number: 1450102)
4D-Nucleofector® X Unit (Lonza, catalog number: AAF-1003B)
Bright-LineTM hematocytometer (Sigma-Aldrich, catalog number: Z359629)
Optional; see steps A2 and A3: BD FACSAria III Cell Sorter (BD Biosciences)
Software and datasets
CrispRGold (free) (https://crisprgold.mdc-berlin.de/) or CRISPOR (free) (http://crispor.tefor.net/)
ICE CRISPR Analysis Tool (Synthego) (free)
https://www.synthego.com/products/bioinformatics/crispr-analysis
GeneOptimizer Algorithm (GeneArt Gene Synthesis Services, Thermo Fisher Scientific) (free)
https://www.thermofisher.com/de/de/home/life-science/cloning/gene-synthesis/geneoptimizer.htmL
FlowJo (v.10, FlowJo, BD Biosciences) (commercial analysis software, requires a license)
ApE (A Plasmid Editor) (free) (https://jorgensen.biology.utah.edu/wayned/ape/)
Procedure
文章信息
稿件历史记录
提交日期: May 8, 2024
接收日期: Aug 16, 2024
在线发布日期: Sep 25, 2024
出版日期: Oct 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Li, X., Chu, V. T., Kocks, C. and Rajewsky, K. (2024). Expansion and Precise CRISPR-Cas9 Gene Repair of Autologous T-Memory Stem Cells from Patients with T-Cell Immunodeficiencies. Bio-protocol 14(20): e5085. DOI: 10.21769/BioProtoc.5085.
- Li, X., Wirtz, T., Weber, T., Lebedin, M., Lowenstein, E. D., Sommermann, T., Zach, A., Yasuda, T., de la Rosa, K., Chu, V. T., et al. (2024). Precise CRISPR-Cas9 gene repair in autologous memory T cells to treat familial hemophagocytic lymphohistiocytosis. Sci Immunol. 9(92): eadi0042.
分类
免疫学 > 免疫疗法
细胞生物学 > 细胞工程 > CRISPR-cas9
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link