- EN - English
- CN - 中文
Sorghum bicolor Extracellular Vesicle Isolation, Labeling, and Correlative Light and Electron Microscopy
高粱细胞外囊泡的分离、标记及相关光电显微技术
(*contributed equally to this work) 发布: 2024年10月05日第14卷第19期 DOI: 10.21769/BioProtoc.5083 浏览次数: 2039
评审: Xiaofei LiangAnuradha SinghMasayoshi Nakamura
Abstract
Extracellular vesicles are membrane-bound organelles that play crucial roles in intercellular communication and elicit responses in the recipient cell, such as defense responses against pathogens. In this study, we have optimized a protocol for isolating extracellular vesicles (EVs) from Sorghum bicolor apoplastic wash. We characterized the EVs using fluorescence microscopy and correlative light and electron microscopy.
Key features
• Allows the isolation of extracellular vesicles from the monocot plant Sorghum bicolor.
• Labels isolated extracellular vesicles with fluorescent dyes for easy characterization with light microscopy.
• Validates dye labeling and further characterizes extracellular vesicles using a correlative light and electron microscopy approach.
Keywords: Sorghum bicolor (高粱)Background
Extracellular vesicles (EVs) are membrane-bound organelles secreted by cells [1,2]. Many studies have shown that EVs are packaged with RNA, lipids, proteins, and other metabolites; these EV contents play an important role in mediating intercellular signaling [1–3].
The observation of plant and fungal EVs deposited at fungal infection sites suggested a possible mediation of the plant–pathogen interaction by EVs [4–6]. The notion of EVs mediating plant–pathogen interactions is supported by multiple studies showing enhanced plant EVs secretion after fungal infection, silencing of host plant’s immunity genes by sRNA packaged in fungal EVs, and modulation of fungal gene expression by host plant mRNAs [6–12]. Proteomic analysis of isolated plant EVs revealed they contain plant defense proteins [13–15], and a non-canonical secretory pathway has been proposed as the route for secretion of defense factors and virulence factors by the plants and fungal pathogens, respectively [16,17]. However, despite plant EVs having been described since the 1960s [5], methods for their isolation were only developed fairly recently for eudicot plants. Eudicot plant EVs have been largely isolated from the apoplastic wash of leaves through differential centrifugation, immuno-affinity capture, and density gradient centrifugation [3,15,18].
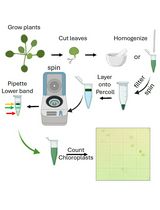
Here, we provide a detailed protocol that we used previously [19] for the isolation and characterization of EVs from the monocot plant Sorghum bicolor. To detect EVs by fluorescence microscopy, we designed a method for staining the EVs using an esterified dye, Calcein AM Green, and a lipophilic dye, Potomac Gold. To further characterize EVs, we developed a correlative light and electron microscopy method to examine the morphology of EVs stained with our fluorescent labeling approach. The new methods developed here complement standard EV characterization methods, such as nanoparticle tracking analysis and transmission electron microscopy.
Materials and reagents
Pro-mix BK55 soil (Premier Tech Horticulture, catalog number: 5060055RG)
6” standard pot (Grower’s, catalog number: 03EJ-SP600)
Sorghum bicolor seeds (BTx623 inbred line)
Scissors (W.B. Mason, catalog number: ACM13529)
Parafilm®, 4 in. × 250 ft (Bemis, catalog number: PM999)
Pyrex dish (Corning, catalog number: 3160100)
60 mL syringe (BD medical, catalog number: BD301036)
8-inch half-round file (Harbor Freight, catalog number: 96629)
Open-top thick-wall polypropylene tubes, 50 mL, 29 mm × 104 mm (Beckman Coulter, catalog number: 357007)
250 mL Nalgene bottles, (Millipore Sigma, catalog number: B9157)
2-(morpholin-4-yl)ethane-1-sulfonic acid (MES) hydrate (Sigma-Aldrich, catalog number: M8250)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: SS7653)
Calcium chloride (CaCl2) (Sigma-Aldrich, catalog number: C1016)
1 L Glass beaker (Pyrex, catalog number: 100-1L-PK)
Miracloth 475855-1R (Millipore, catalog number: 3446249)
1 M Tris-Hydrochloride (Tris-HCl), pH 7.5 (Fisher Bioreagents, catalog number: BP1757-500)
KimwipesTM (Kimberly-Clark, catalog number: 34120)
25 ml serological pipettes (Thermo Fisher Scientific, catalog number: 170357N)
OptiPrep density gradient (60% iodixanol) (Sigma-Aldrich, catalog number: D1556)
Thin-walled ultra-clear centrifuge tubes (14 mm × 89 mm) (Beckman Coulter, catalog number: 344059)
Calcein Green, AM (Thermo Fisher Scientific, catalog number: C34852)
Potomac Gold (Lavis Lab, HHMI Janelia)
Poly-L-lysine (Sigma-Aldrich, catalog number: P4707)
Trypan blue (Sigma-Aldrich, catalog number: T6146)
HPLC grade water (Thermo Fisher Scientific, catalog number: 022934)
Microscopes slides, 75 mm × 25 mm × 1 mm (Thermo Fisher Scientific, catalog number: 12-544-2)
Cover glass, 22 mm × 22 mm, No. 1.5 (Thermo Fisher Scientific, catalog number: 12541B)
Secure seal spacer, 9 mm diameter (Thermo Fisher Scientific, catalog number: S24737)
Isopropyl alcohol (IPA) (Millipore Sigma, catalog number: W292907)
5% (3-aminopropyl)triethoxysilane (APTES) (Millipore Sigma, catalog number: 440140-100ML)
25% Glutaraldehyde (Electron Microscopy Supplies, catalog number: 16220)
1.5 mL Polypropylene microfuge tubes (Beckman Coulter, catalog number: 357448)
Ethanol 200 proof (Thermo Fisher Scientific, catalog number: T038181000)
Molecular-grade ethanol (Electron Microscopy Sciences, catalog number: 15055)
HPLC-grade ethanol (Thermo Scientific Chemicals, catalog number: AS611050040)
Ibidi Grid-50 cover glasses (Ibidi, catalog number: 10817)
Attofluor cell chamber (Thermo Fisher, catalog number: A7816)
Aluminum mounts to fit in SEM (Electron Microscopy Sciences, catalog number: 75185)
Ultra-smooth carbon adhesive tabs, 25 mm diameter (Electron Microscopy Sciences, catalog number: 778727-25)
PELCO conductive silver paint (Ted Pella, Inc, catalog number: 16062-15)
Wash-N-DryTM coverslip rack (VWR, catalog number: 490007-150)
Dimethyl sulfoxide (DMSO) (Sigma-Aldrich, catalog number: D4540)
Solutions
Vesicle isolation buffer (VIB) (see Recipes)
Calcein AM stain (see Recipes)
Potomac gold stain (see Recipes)
Trypan blue (see Recipes)
Glutaraldehyde fixative (see Recipes)
Ethanol dehydration solutions (see Recipes)
Recipes
Vesicle isolation buffer (VIB)
20 mM MES
2 mM CaCl2
0.1 M NaCl
Adjust pH to 6.0. Autoclave at 121 °C for 30 min
Calcein AM Stain
Resuspend 50 µg of Calcein Green, AM in 50 µL of DMSO.
Dilute to 1 µM in HPLC water.
Potomac gold stain
Resuspend 100 µg of Potomac Gold in 100 µL of DMSO.
Make 5 µL aliquots.
Dilute to 1 µM in HPLC water.
Trypan blue
Make a 100 nM stock solution in HPLC water.
Glutaraldehyde fixative
Make fresh.
Filter 50 mL of 20 mM glutaraldehyde, pH 7.5, through a 0.2 µm filter into a 50 mL conical tube.
Make 1% and 2% glutaraldehyde in filtered 20 mM Tris-HCl, pH 7.5.
Ethanol dehydration solutions
Make a 25%, 50%, 75%, and 95% ethanol concentration series by diluting HPLC-grade ethanol in HPLC water.
Equipment
Light meter (Li-Cor, model: Quantum LI-190-R and LI-350)
Vacuum pump (Cole Parmer Welch, model: 2585B-50)
Vacuum desiccator chamber (Millipore Sigma, model: F42400-2221, catalog number: BAF424002221)
Avanti J-20 centrifuge (Beckman Coulter, catalog number: 368608)
JA-14 fixed-angle rotor (Beckman Coulter, model: JA-14, catalog number: 339247)
JA-17 fixed angle rotor (Beckman Coulter, model: JA-17, catalog number: 369691)
OptimaTM L-90k ultracentrifuge (Beckman Coulter, catalog number: 2043-30-1191)
SW 41 Ti rotor, swinging bucket, titanium, 6 × 13.2 mL (Beckman Coulter, catalog number: 331362)
OptimaTM MAX tabletop ultracentrifuge (Beckman Coulter, catalog number: 393315)
TLA 55 fixed-angle rotor package (Beckman Coulter, catalog number: 366725)
Total internal reflection fluorescence microscope (TIRF) (Oxford Instruments, model: Andor Dragonfly 600)
Note: Other sensitive microscopes can be used; however, TIRF and spinning disk confocal microscopes are recommended. Here, we used Borealis TIRF on an Andor Dragonfly 600 microscope with an Andor Zyla 4.2 PLUS sCMOS camera and a Leica Plan Apo 63× oil immersion TIRF objective (numerical aperture, 1.47). We used a 2,000mW, 488nm excitation laser, and 521/38nm bandpass (BP) filter for Calcein AM and a 1,000mW, 561nm excitation laser, and 594/43nm BP filter for Potomac Gold.
Stainless steel tweezers (Tronex, catalog number: SA316L)
Cover glass drying rack (Millipore Sigma, catalog number: Z743684)
Sonicator (Elma, catalog number: 51000065766)
Critical point dryer (Tousimis, model: Autosamdri®-815B, Series A, catalog number: 8780B)
Apreo VolumeScopeTM scanning electron microscope with Maps 3.9 software
Software and datasets
ImageJ
Procedure
文章信息
稿件历史记录
提交日期: Jun 7, 2024
接收日期: Aug 19, 2024
在线发布日期: Sep 10, 2024
出版日期: Oct 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Adekanye, D., Chaya, T. and Caplan, J. L. (2024). Sorghum bicolor Extracellular Vesicle Isolation, Labeling, and Correlative Light and Electron Microscopy. Bio-protocol 14(19): e5083. DOI: 10.21769/BioProtoc.5083.
- Chaya, T., Banerjee, A., Rutter, B. D., Adekanye, D., Ross, J., Hu, G., Innes, R. W. and Caplan, J. L. (2023). The extracellular vesicle proteomes of Sorghum bicolor and Arabidopsis thaliana are partially conserved. Plant Physiol. 194(3): 1481–1497.
分类
植物科学 > 植物细胞生物学 > 细胞器分离
植物科学 > 植物生物化学 > 脂质
生物化学 > 脂质 > 胞外脂质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link