- EN - English
- CN - 中文
Alternative Method for Obtaining Human-Induced Pluripotent Stem Cell Lines and Three-Dimensional Growth: A Simplified, Passage-Free Approach that Minimizes Labor
获得人诱导多能干细胞系及三维培养的替代方法:一种简化且无需传代的低劳动量方法
发布: 2024年10月05日第14卷第19期 DOI: 10.21769/BioProtoc.5081 浏览次数: 1802
评审: Alessandro DidonnaMasashi ToyodaGiovanna Piovani
Abstract
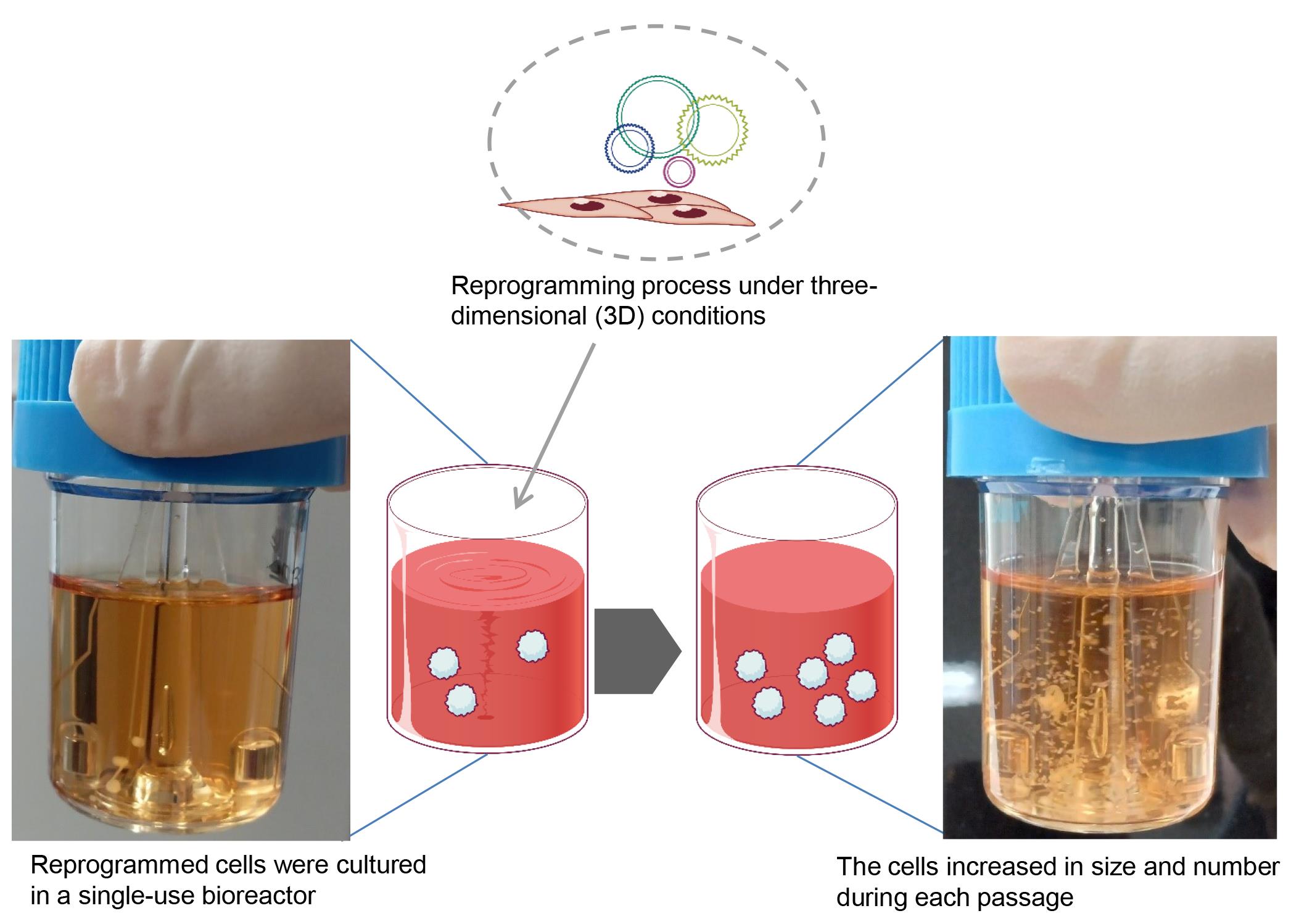
Induced pluripotent stem cells (iPSCs) hold significant promise for numerous applications in regenerative medicine, disease modeling, and drug discovery. However, the conventional workflow for iPSC generation, with cells grown under two-dimensional conditions, presents several challenges, including the need for specialized scientific skills such as morphologically assessing and picking colonies and removing differentiated cells during the establishment phase. Furthermore, maintaining established iPSCs in three-dimensional culture systems, while offering scalability, necessitates an enzymatic dissociation step for their further growth in a complex and time-consuming protocol. In this study, we introduce a novel approach to address these challenges by reprogramming somatic cells grown under three-dimensional conditions as spheres using a bioreactor, thereby eliminating the need for two-dimensional culture and colony picking. The iPSCs generated in this study were maintained under three-dimensional conditions simply by transferring spheres to the next bioreactor, without the need for an enzymatic dissociation step. This streamlined method simplifies the workflow, reduces technical variability and labor, and paves the way for future advancements in iPSC research and its wider applications.
Key features
• Establishment of induced pluripotent stem cells in a three-dimensional environment.
• Maintenance and cryopreservation of iPSCs without the need for a dissociation step.
Keywords: Bioreactor (生物反应器)Graphical overview
Background
Induced pluripotent stem cells (iPSCs), derived from somatic cells, exhibit characteristics similar to those of embryonic stem cells. This makes them valuable tools in regenerative medicine and disease modeling [1]. However, the process of generating iPSCs is fraught with challenges, especially during the establishment phase, which requires specialized technical skills [2,3]. During the reprogramming phase, partially reprogrammed cells, re-differentiated cells, and non-reprogrammed cells all typically proliferate. However, a common issue is trying to attain spontaneous differentiation until cells achieve stable pluripotent states. It is therefore necessary to select and isolate iPSC colonies with optimal morphology during the initial passages. Such complexities contribute to the difficulties and inherent variability of iPSC cultures, which often depend on the technician's skills.
Traditionally, iPSCs are maintained under two-dimensional conditions using a specialized medium and feeder cells or an extracellular matrix reagent. This approach hampers scalability, which is crucial for iPSC downstream applications. Recently, the maintenance of iPSC lines under three-dimensional (3D) conditions has been shown to offer scalability. This has been achieved using agitation rotor machines or bioreactors [4,5]. However, the enzymatic dissociation step required for single-cell cultures under 3D conditions is both time-consuming and complex [4,6].
In particular, the generation of personalized iPSCs for use in regenerative medicine necessitates a simplified and streamlined workflow for iPSC studies. This workflow should minimize human handling during cell reprogramming, the maintenance of iPSCs, and their differentiation into specific cell types. Furthermore, it is also important that this simplified method can be scaled up to produce adequate cell mass.
To address these issues and reduce variability and workload for technicians, a more streamlined and convenient iPSC workflow is needed. We have developed the protocol presented here to enable iPSC generation under 3D culture conditions, thereby eliminating the need for a colony-picking step [7]. Moreover, our 3D culture system allows for the maintenance and cryopreservation of iPSCs without the need for enzymatic cell dissociation steps, resulting in a significantly simplified and easy-to-follow workflow. Given the compact size of the bioreactor used in this protocol (approximately 148 mm wide × 267 mm deep × 33 mm high with a maximum capacity of six samples at the same time), our protocol holds promise for the full automation of iPSC generation and maintenance in the future.
Materials and reagents
Biological materials
Stealth RNA vector (SRV)TM iPSC vector; SRV iPS-2 vector (encoding OCT4, KLF4, SOX2, and C-MYC; TOKIWA-Bio Inc., Ibaraki, Japan, catalog number: S1011694A) based on the Sendai virus
SRVTM iPSC vector; SRV iPS-4 vector (carrying OCT4, KLF4, SOX2, C-MYC, NANOG, and LIN28; TOKIWA-Bio Inc., catalog number: S1011696A)
Adipose-derived mesenchymal stem cells (Lonza Bioscience, Walkersville, MD, USA, catalog number: PT5006)
Human peripheral blood mononuclear cells (PBMCs) (e.g., derived from healthy donors or patients)
Reagents
MesenPRO RSTM medium (Thermo Fisher Scientific, catalog number: 12746012)
KBM 501 medium (Kohjin Bio, catalog number: 16025015)
Fetal bovine serum (FBS) (Thermo Fisher Scientific, catalog number: 10270-106)
TrypLE Select (Thermo Fisher Scientific, catalog number: 12563-011)
Dulbecco's phosphate-buffered saline (DPBS) (Thermo Fisher Scientific, catalog number: 14190144)
UltraPureTM 0.5 M EDTA, pH 8.0 (Thermo Fisher Scientific, catalog number: 15575020)
StemScaleTM PSC suspension medium (StemScale) (Thermo Fisher Scientific, catalog number: A4965001)
DAPT, gamma-secretase inhibitor (Abcam, catalog number: ab120633)
EPZ004777 (iDOT1L) (Tocris, catalog number: 5567)
Y27632 (FUJIFILM Wako Pure Chemical Corporation, catalog number: 036-24023)
STEM-CELLBANKER® GMP grade (ZENOAQ, catalog number: 11924)
RNeasy Mini kit (Qiagen, catalog number: 74904)
SuperScript IV VILO Master Mix (Thermo Fisher Scientific, catalog number: 11756050)
TaKaRa Ex Taq DNA Polymerase (Takara Bio Inc., catalog number: RR001B)
Primer pair for β-ACTIN and SRV (e.g., Sigma custom oligo; oligo sequence is below)
β-ACTIN (131 bp)
Forward: 5-TCCCTGGAGAAGAGCTACG-3
Reverse: 5-GTAGTTTCGTGGATGCCACA-3
SRV (500 bp)
Forward: 5-ATATGGAGTACGAGAGGACC-3
Reverse: 5-CCTCAGGTTGGAGAGAGTCA-3
Agarose gel (e.g., Nacalai Tesque Inc., catalog number: 01158-85)
Ethidium bromide (e.g., FUJIFILM Wako Pure Chemical Corporation, catalog number: 315-90051)
Solutions
Cell dissociation solution (see Recipes)
PBMC medium (see Recipes)
Reprogramming medium (see Recipes)
Recipes
Cell dissociation solution
Reagent Final concentration Amount TrypLE Select 0.5× 25 mL DPBS 0.5× 25 mL EDTA 0.25 mM 25 µL Total 50 mL PBMC medium
Reagent Final concentration Amount KBM 501 medium 90% 45 mL FBS 10% 5 mL Total 50 mL Reprogramming medium
Reagent Final concentration Amount StemScale n/a 100 mL DAPT 5 µM EPZ004777 3 µM Y27632 10 µM Total 100 mL n/a, not applicable
Laboratory supplies
Microtube 1.5 mL (e.g., Sumitomo Bakelite Co., Ltd., catalog number: MS-4265M)
15 mL centrifuge tube (e.g., IWAKI, catalog number: 2324-015)
30 mL disposable bioreactor (ABLE Biott, catalog number: ABBWVS03A-6)
6-well dish (e.g., IWAKI, catalog number: 3810-006N)
96-well U-bottom dish (e.g., Corning, catalog number: 7007)
PCR tube (e.g., Eppendorf, catalog number: 30124359)
Cryotube (e.g., Thermo Fisher Scientific, catalog number: 377267)
Equipment
CO2 incubator (e.g., Thermo Fisher Scientific, catalog number: 3110, FormaTM series II)
-80 °C freezer (e.g., Panasonic, catalog number: MDF-U33V-PJ)
Centrifuge (e.g., TOMY, catalog number: NIX521)
Bioreactor magnetic stir system base (Able Corp. & Biott Co., catalog number: ABBWBP03N0S-6)
Laser microscope (e.g., Keyence, catalog number: BZ-X810)
Thermal cycler (e.g., Thermo Fisher Scientific, catalog number: 4484073)
Cell counter (e.g., Beckman Coulter, Vi-CELL XR Cell Viability Analyzer System)
Procedure
文章信息
稿件历史记录
提交日期: Jun 12, 2024
接收日期: Aug 22, 2024
在线发布日期: Sep 10, 2024
出版日期: Oct 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Tsukamoto, M., Kawasaki, T., Umezawa, A. and Akutsu, H. (2024). Alternative Method for Obtaining Human-Induced Pluripotent Stem Cell Lines and Three-Dimensional Growth: A Simplified, Passage-Free Approach that Minimizes Labor. Bio-protocol 14(19): e5081. DOI: 10.21769/BioProtoc.5081.
分类
干细胞 > 多能干细胞 > 基于细胞的分析方法
干细胞 > 多能干细胞 > 细胞诱导
细胞生物学 > 细胞分离和培养 > 低温贮存
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link