- EN - English
- CN - 中文
Acutely Modifying Phosphatidylinositol Phosphates on Endolysosomes Using Chemically Inducible Dimerization Systems
使用化学诱导二聚化系统快速调控内溶酶体上的磷脂酰肌醇磷酸盐
发布: 2024年10月05日第14卷第19期 DOI: 10.21769/BioProtoc.5078 浏览次数: 1573
评审: Ralph Thomas BoettcherAlexandros C KokotosAnonymous reviewer(s)
Abstract
Phosphoinositides are rare membrane lipids that mediate cell signaling and membrane dynamics. PI(4)P and PI(3)P are two major phosphoinositides crucial for endolysosomal functions and dynamics, making them the lipids of interest in many studies. The acute modulation of phosphoinositides at a given organelle membrane can reveal important insights into their cellular function. Indeed, the localized depletion of PI(4)P and PI(3)P is a viable tool to assess the importance of these phosphoinositides in various experimental conditions. Here, we describe a live imaging method to acutely deplete PI(4)P and PI(3)P on endolysosomes. The depletion assay utilizes the GAI-GID1 or the FRB-FKBP inducible dimerization system to target the catalytic domain of the PI(4)P phosphatase, Sac1, or the PI(3)P phosphatase domain of MTM1 to the endolysosome for localized depletion of these phosphoinositides. By using the fluorescently tagged biosensors, 2xP4M and PX, we can validate and monitor the depletion of PI(4)P and PI(3)P, respectively, on endolysosomes in real-time. We discuss a method for normalizing the fluorescence measurements to appropriate the relative amount of these phosphoinositides in the organellar membranes (endolysosomes), which is required for monitoring PI(4)P or PI(3)P levels during the acute depletion assay. Since the localization of the dimerization partners is specified by the membrane targeting signal, our protocol will be useful for studying the signaling and functions of phosphoinositides at any membrane.
Key features
• Acute depletion and real-time monitoring of PI(3)P and PI(4)P on the endolysosomal membrane using chemically inducible dimerization systems.
• Modifiable and adaptable to modulate other phosphoinositides on different organellar membranes.
Keywords: Phosphoinositides (磷酸肌醇类)Graphical overview
Background
Phosphoinositides, such as PI(4)P and PI(3)P, are phosphorylated derivatives of phosphatidylinositol, a phospholipid found in the cytoplasmic leaflet of eukaryotic membranes. The inositol ring of the phosphatidylinositol headgroup can be phosphorylated by specific kinases, and dephosphorylated by phosphatases, in position 3, 4, and 5 for a total of seven different phosphoinositides [1]. Phosphoinositides are key signaling lipids that regulate organelle membrane identity and dynamics. They notably play major roles in orchestrating various endolysosomal functions and dynamic events such as tubulation and fission, which are required for the reformation of lysosomes at the end of the autophagic and endocytic processes [2–5].
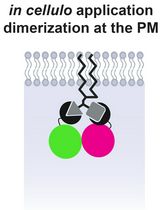
One elegant way to investigate the role of phosphoinositides in organelle biology is to modulate their levels at a given membrane. This can be done by specifically targeting phosphoinositide kinases or phosphatases to an organelle of interest. Targeting can be achieved by fusing the catalytic domain of kinases or phosphatases to a membrane-targeting domain (typically a transmembrane domain) of a resident membrane protein of the desired organelle. This allows for the constitutive depletion of the phosphoinositides upon the expression of the protein. However, to allow for temporal and acute regulation of the phosphoinositides species, the catalytic domain can be targeted using a chemically induced dimerization system. Here, one part of the dimer is anchored at a membrane of interest by fusing it to a transmembrane protein/targeting sequence, and the other is coupled to a cytosolic version of a phosphatase or kinase. Upon the addition of chemical inducers of dimerization, the binding affinity between the two-part dimerization system increases drastically, promoting the recruitment of the cytosolic part to the membrane-anchored part of the dimer. As a result, the recruited kinases and phosphatases actively phosphorylate and dephosphorylate the phosphoinositide substrate, respectively, depleting them from the organellar membrane.
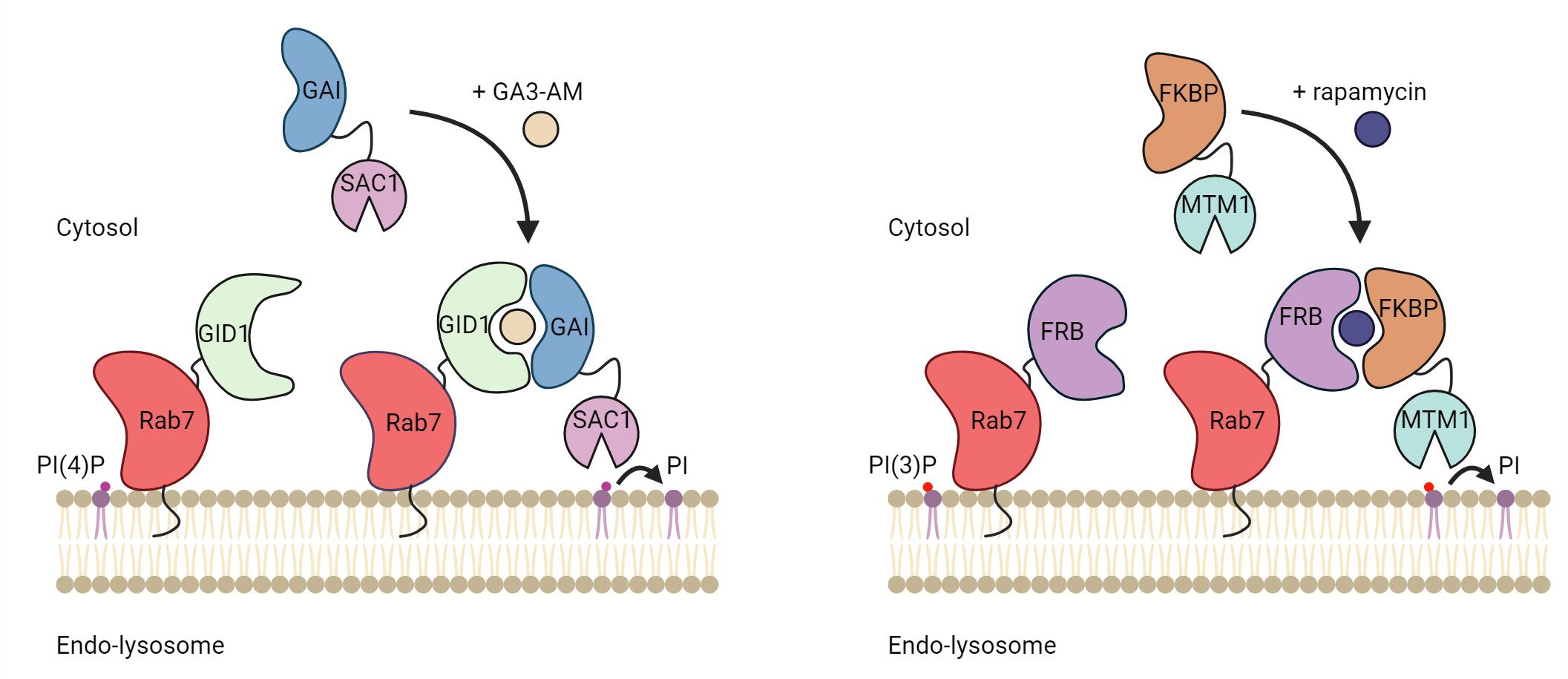
In addition, the level of specific phosphoinositides at membranes in cells can be evaluated by the ectopic expression of genetically encoded phosphoinositides biosensors coupled to a fluorescent protein, such as GFP or mCherry. Over the last 20 years, high-affinity binding domains for specific phosphoinositides have been identified and used as biosensors to detect different phosphoinositide species [6]. Here, we present a protocol utilizing chemically induced dimerization systems to deplete specific phosphoinositides at endolysosomes and biosensors to detect their depletion. We describe how to use two different dimerization systems in cells, the GAI-GID1 and the FRB-FKBP, to deplete PI(4)P and PI(3)P, respectively, at Rab7-positive endolysosomes (hereafter referred to as endolysosomes). However, any of the two dimerization systems can be used to deplete the phosphoinositides on any other membrane-bound organelle of interest. In this protocol, we discuss the experimental setup to express these systems in mammalian cells and how to perform image acquisition and analysis to validate the depletion of PI(4)P or PI(3)P.
The FRB-FKBP dimerization system was invented in the 90’s [7] and relies on the dimerization of FRB and FKBP in the presence of rapamycin, a cell-permeant chemical most famous for its inhibitory effect on mTORC1 (mammalian target of rapamycin complex 1). The FRB and FKBP domains are relatively small (~10 kDa) and thus can be efficiently transfected and expressed, reducing the likeliness of impacting the function of the protein of interest fused to these domains. However, using rapamycin in the FRB-FKBP dimerization system may cause undesirable changes in certain mammalian biological processes such as autophagy and nutrient-sensing pathways, notably due to its effect on mTORC1. To circumvent this problem, rapalogs (rapamycin analogs) can be used to alleviate this cross-reactivity [8]. Another way to mitigate the complications of rapamycin is to use the plant-derived GA3-AM-inducible GAI-GID1 dimerization system since there is no known mammalian target of GA3-AM [9]. However, unlike the FRB-FKBP system, both GAI and GID1 are medium-sized protein domains (~59 and ~40 kDa, respectively) which may impede the efficient introduction or expression of proteins. Control experiments can then be performed to validate that tagging with dimerizing proteins does not impair the localization or the function of the protein of interest. Here, we present a protocol to target PI(4)P or PI(3)P phosphatases to endolysosomes and to validate phosphoinositides depletion using genetically encoded PI(4)P or PI(3)P biosensors.
Materials and reagents
Cell line
HeLa cells from ATCC (CCL-2) [here, HeLa cells are used. However, this can be used in any cell lines. The only difference may be the system to insert the necessary plasmid into the cell (transfections vs. transduction vs. electroporation, etc.)]
Plasmids (Table 1)
Table 1. Plasmids used in this protocol
| Plasmid | Addgene number | Reference |
|---|---|---|
| iRFP-GID1-Rab7 | Levin-Konigsberg et al. [10] | |
| CFP-GAI-Sac1 | Boutry and Kim [11] | |
| CFP-GAI-Sac1 C392S | Boutry and Kim [11] | |
| mCherry-2xP4M | Levin-Konigsberg et al. [10] | |
| Lamp1-mCherry | 45147 | Van Engelenburg and Palmer [12] |
| iRFP-FRB-Rab7 | 51613 | Hammond et al. [13] |
| mCherry-FKBP-MTM1 | 51614 | Hammond et al. [13] |
| mCherry-FKBP | 67514 | Varnai et al. [14] |
| PX-GFP | 19010 | Kanai et al. [15] |
| Lamp1-GFP | 16290 | Minin et al. [16] |
Reagents
DMEM, 4.5 g/L glucose, supplemented with L-glutamine and sodium pyruvate (Wisent, catalog number: 319-005 CL)
Fetal bovine serum (FBS) (Wisent, catalog number: 098150)
Trypsin-EDTA (0.05% Trypsin, 0.53 mM EDTA) (Wisent, catalog number: 325542105)
D-PBS (Wisent, catalog number: 311-45 CL)
Neon electroporation kit 100 μL (Invitrogen, catalog number: MPK10096)
Resuspension buffer R
Electrolytic buffer E2
Neon electroporation tips (100 µL)
Electroporation tubes
Rapamycin (Sigma-Aldrich, catalog number: 553210)
GA3-AM (Sigma-Aldrich, catalog number: SML1959)
Laboratory supplies
T75 cm2 cell culture flasks (Sarstedt Inc, catalog number: 50-809-261)
4-well Lab-Tek II chambered cover glass (Nunc, catalog number: 155382)
15 mL conical tubes
1.5 mL Eppendorf tubes
Equipment
Micropipette (P1000, P200, and P10)
Centrifuge with adaptors for 15 mL conical tubes
Tissue culture CO2 incubator
Standard inverted light microscope
Hemocytometer with cover glass
Inverted microscope (Plan-Apochromat 63×/1.40 oil objective) (Zeiss, model: Airyscan 2 LSM 980) with temperature- and CO2-controlled imaging chamber
Neon NxT Electroporation System (electroporation device, electroporation pipette and pipette station, electroporation tube, and tube chamber)
Software and datasets
ImageJ [17] or Fiji software [18] (both open source)
Procedure
文章信息
稿件历史记录
提交日期: May 25, 2024
接收日期: Aug 7, 2024
在线发布日期: Sep 13, 2024
出版日期: Oct 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Yap, W. S., Kim, P. K. and Boutry, M. (2024). Acutely Modifying Phosphatidylinositol Phosphates on Endolysosomes Using Chemically Inducible Dimerization Systems. Bio-protocol 14(19): e5078. DOI: 10.21769/BioProtoc.5078.
- Boutry, M., DiGiovanni, L. F., Demers, N., Fountain, A., Mamand, S., Botelho, R. J. and Kim, P. K. (2023). Arf1-PI4KIIIβ positive vesicles regulate PI(3)P signaling to facilitate lysosomal tubule fission. J Cell Biol. 222(9): e202205128.
分类
细胞生物学 > 细胞成像 > 活细胞成像
细胞生物学 > 细胞成像 > 共聚焦显微镜
细胞生物学 > 细胞结构 > 细胞器
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link