- EN - English
- CN - 中文
Construction and Application of a Static Magnetic Field Exposure Apparatus for Biological Research in Aqueous Model Systems and Cell Culture
用于生物研究的水模型系统和细胞培养静态磁场暴露装置的构建与应用
发布: 2024年10月05日第14卷第19期 DOI: 10.21769/BioProtoc.5077 浏览次数: 1741
评审: Willy R Carrasquel-UrsulaezYoungchan KimSusovan Chowdhury
Abstract
With the growth of the quantum biology field, the study of magnetic field (MF) effects on biological processes and their potential therapeutic applications has attracted much attention. However, most biologists lack the experience needed to construct an MF exposure apparatus on their own, no consensus standard exists for exposure methods, and protocols for model organisms are sorely lacking. We aim to provide those interested in entering the field with the ability to investigate static MF effects in their own research. This protocol covers how to design, build, calibrate, and operate a static MF exposure chamber (MagShield apparatus), with instructions on how to modify parameters to other specific needs. The MagShield apparatus is constructed of mu-metal (which blocks external MFs), allowing for the generation of experimentally controlled MFs via 3-axial Helmholtz coils. Precise manipulation of static field strengths across a physiologically relevant range is possible: nT hypomagnetic fields, μT to < 1 mT weak MFs, and moderate MFs of several mT. An integrated mu-metal partition enables different control and experimental field strengths to run simultaneously. We demonstrate (with example results) how to use the MagShield apparatus with Xenopus, planarians, and fibroblast/fibrosarcoma cell lines, discussing the modifications needed for cell culture systems; however, the apparatus is easily adaptable to zebrafish, C. elegans, and 3D organoids. The operational methodology provided ensures uniform and reproducible results, affording the means for rigorous examination of static MF effects. Thus, this protocol is a valuable resource for investigators seeking to explore the intricate interplay between MFs and living organisms.
Key features
• A comprehensive roadmap, suitable for undergraduate to advanced researchers, to construct an apparatus for in vitro and in vivo experiments within uniform static magnetic fields.
• Designed to fit inside standard incubators to accommodate specific environmental conditions, such as with cell culture, in addition to stand-alone operation at room temperature.
• Requires two DC power supplies and 3D printer access for the Helmholtz coils, Plexiglass and mu-metal foil for the partition, and a milli/Gaussmeter for calibration.
• Requires ordering a custom mu-metal shell from a commercial resource (using provided schematics), where lead times for delivery can vary from 2 to 4 months.
Keywords: Quantum biology (量子生物学)Graphical overview
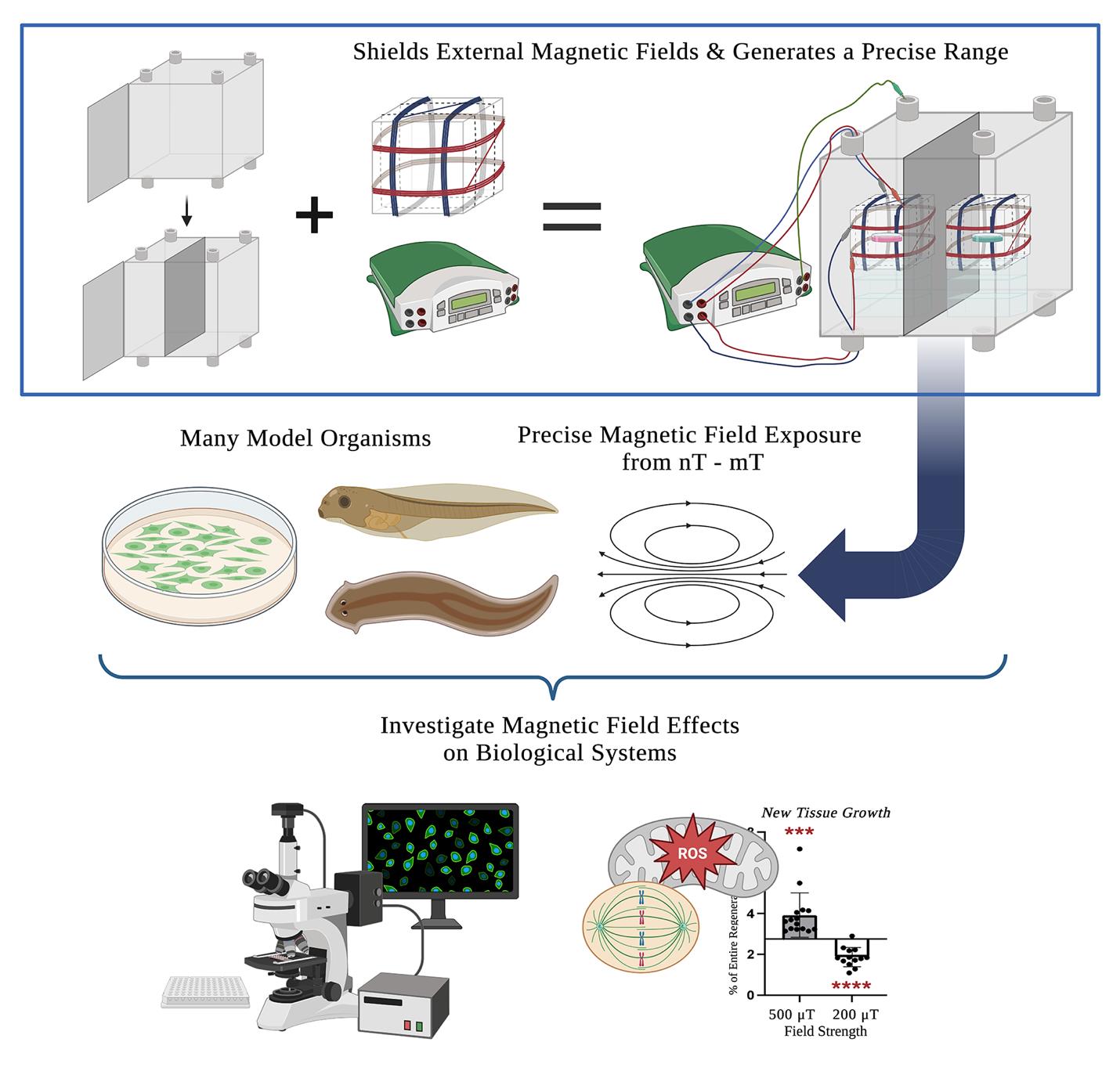
MagShield apparatus. This protocol offers a detailed guide on the construction and use of a static magnetic field exposure chamber (top right) for the investigation of magnetic field effects on biological processes. It consists of a mu-metal enclosure (top left), the design and calibration of 3-axial Helmholtz coils (top middle), and considerations for its use with aquatic (Xenopus embryos and larvae and adult planarians), cell culture model systems, and near-zero fields.
Background
Life evolved within the Earth’s natural geomagnetic field, which varies from 25 to 65 μT. However, in the modern environment, anthropogenic magnetic fields (MFs) generated by electronic devices and high-voltage power lines are now ubiquitous [1,2]. Exposures range from very low (e.g., 0.1–3 μT) for household appliances to extremely high (from 1.5 to 7 T) for clinical magnetic resonance imaging (MRI) devices [3–7]. With emerging technologies, new avenues for human exposure to non-ionizing radiation are continually introduced [8,9]. Additionally, the number of diagnostic and therapeutic uses for MFs is growing. The widely used MRI diagnostic relies on static MFs to provide detailed anatomical and physiological information [6,7]. Therapeutically, electromagnetic fields (EMFs) are used for pain management during rehabilitation and with musculoskeletal diseases such as neuropathy and fibromyalgia [10–14]. Research has demonstrated that exposure to even weak MFs can affect biological systems by altering free radical formation—believed to result from MF interactions with spin dynamics [15–21]. Weak MFs have been shown to change reactive oxygen species (ROS) signaling and alter cellular outcomes in vivo [19,22], and thus they represent a potential means to control host defense/immunological responses, regenerative stem cell proliferation, and cancer progression [23–28]. Despite increased interest in uncovering the underlying mechanisms, standardized methods for experimental exposure are lacking and few model systems have been used for in vivo studies (for a recent review, see [29]).
Using this protocol, the authors (a collaboration of engineers, developmental biologists, and geneticists) have successfully investigated MF effects in several different model systems. In vitro, we showed that exposure to static MFs of 0.5 μT and 600 μT MFs inhibited HT-1080 fibrosarcoma cell growth in culture, while 300 and 400 μT increased growth [16,17]. In vivo, we demonstrated that static weak MF exposure of regenerating planarians was able to manipulate stem cell proliferation and gene expression (where 200 μT decreased and 200 μT increased new tissue growth) via changes in superoxide accumulation after injury [19,22]. Our current efforts aim to investigate hypomagnetic field (nT) effects in the vertebrate model organism, Xenopus laevis. The use of a single standardized exposure protocol increases the ease of mechanistic comparisons across organisms and prevents confounding effects from the variance in MFs arising from incubators and standard laboratory equipment (hypothesized to contribute to experimental reproducibility issues even in non-MF experiments, which themselves could benefit from this type of controlled environment). This protocol provides step-by-step instructions on assembling a single 16 (w) × 16 (h) × 16 (d) in (40.64 cm × 40.64 cm × 40.64 cm) mu-metal MagShield apparatus for static MF exposure assays using 60 mm Petri dishes or 96-well plates, with a partition and two 3-axial Helmholtz coils to run controls and experiments concurrently. We focus here on the room temperature aquatic model systems Xenopus and planarians, as well as temperature-sensitive cell culture requiring placement in standard CO2 incubators with internal dimensions of 18.5 (w) × 23.9 (h) × 22.7 (d) in (46.99 cm × 60.71 cm × 57.66 cm). However, the apparatus could be easily adapted to other model systems (including zebrafish, C. elegans, Drosophila, and organoids), as well as extremely low–frequency EMF exposure.
Materials and reagents
Mu-metal enclosure (to block external MFs), ordered commercially using provided schematics (Mu-Shield Company, custom order). Enclosure inner dimensions: 16 (w) × 16 (d) × 16 (h) in (40.64 cm × 40.64 cm × 40.64 cm). Mu-metal thickness: 0.125 in (3.175 mm). See also Notes 1 and 2
Mu-metal partition (in order to run controls and experiments at different field strengths simultaneously):
Plexiglass sheet (Marketing Holders, ASIN: B08G5DD77N); dimensions: 16 (w) × 16 (h) in (same size as the side of mu-metal enclosure); sheet thickness: 1/8 in (3.175 mm)
Mu-metal foil with adhesive (PST on 1-side) (Magnetic Shield Corporation, item number: MUT002-8); foil length: 6 ft (1.8288 m); foil width: 8 in (20.32 cm) (need a total of 16 in wide but not sold wider than 8 in); foil thickness: 0.002 in (0.051 mm)
Two 3D printed Helmholtz coil base frames (STL file of 3D model provided as Supplemental File S1). Base frames were printed with 2.85 mm diameter PLA filament using an Ultimaker S3 printer with a 0.25 mm nozzle. Base frame outer dimensions: 14 cm × 14 cm × 14 cm. Distance between zip-tie anchors: 7 cm
Enameled copper wire for electrical applications, 17 AWG/1 lb (Emtel, model: EMTHERM200, ASIN: B08NW3YVL3). Wire length: 161 ft (49.0728 m). Wire thickness: 17 AWG diameter (1.15 mm)
Power leads (banana plug to alligator clip), 4 black and 4 red (for connecting the power supplies to the Helmholtz coils) 14 AWG (Zhenyu, item model number: ZY12091, ASIN: B0BPL4C29T). Lead silicone outside diameter: 3.5 mm. Lead length: 6 ft
Grounding lead (banana plug to alligator clip), 1 green, 18 AWG (DigiKey, Pomona Electronics, part number: 501-2514-ND, product number: EM1166-24-5#). Lead length: 2 ft
Note: The grounding lead does not have to be green, although we highly recommend that you use a separate color from the red/black power leads to clearly delineate your grounding lead.
Optional: For hypomagnetic field exposure, two small corrugated cardboard shipping boxes, 4 in × 4 in × 4 in (10.16 cm in all dimensions) for secondary mu-metal shielding (SUNLPH, ASIN: B09QCXPXY1)
Laboratory supplies
Safety glasses
Gloves
Scissors/metal shears
Ruler or tape measure
Zip-ties (wire management for Helmholtz coils)
Electrical tape (to secure lead connections)
Tape/adhesive (duct tape for the partition and laboratory labeling tape for wires is recommended)
Wire strippers
Wire cutters (if not already a function included with the wire strippers)
Sandpaper (may be needed when fitting Plexiglass partition)
Empty welled plates/Petri dishes (for positioning samples in the exact center of the Helmholtz coils)
Note: The width of the plasticware matters as they must fit in between the two rows of a Helmholtz coil pair (~6 cm). We recommend recycling used plasticware for positioning, such as 6/12/24-welled plates or 60 mm Petri dishes.
Empty pipette tip boxes/welled plates (for positioning the Helmholtz coils in the center of each chamber)
Notes:
The size of the plasticware does not matter for positioning the Helmholtz coils.
We recommend recycling used plasticware for positioning.
Optional: For cell culture/incubator placement, polytetrafluoroethylene (PTFE) tape (such as Teflon tape)
Equipment
Two DC power supplies, 300 W dual output, 30V/5A 30V/5A (Jameco, Mastech, part number: 301947, model: HY3005D-2-R)
Note: The dual output power supply is suitable for Helmholtz coils using x- and y-axes only. To use the z-axis as well, a triple output power supply (such as Mastech HY3005D-3-R, Jameco, part number: 301955) should be used.
DC milli/Gaussmeter (for experiments at 0–199 μT) (AlphaLab, Inc., model: MGM, sensor alignment: standard, bandwidth: 3 Hz)
DC Gaussmeter (for experiments at 200–1,000 μT) (AlphaLab, Inc., model: GM1-HS)
Procedure
文章信息
稿件历史记录
提交日期: May 22, 2024
接收日期: Jul 31, 2024
在线发布日期: Sep 10, 2024
出版日期: Oct 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Vučković, J., Gurhan, H., Gutierrez, B., Guerra, J., Kinsey, L. J., Nava, I., Fitzpatrick, A., Barnes, F. S., Tseng, K. A. and Beane, W. S. (2024). Construction and Application of a Static Magnetic Field Exposure Apparatus for Biological Research in Aqueous Model Systems and Cell Culture. Bio-protocol 14(19): e5077. DOI: 10.21769/BioProtoc.5077.
- Van Huizen, A. V., Morton, J. M., Kinsey, L. J., Von Kannon, D. G., Saad, M. A., Birkholz, T. R., Czajka, J. M., Cyrus, J., Barnes, F. S., Beane, W. S., et al. (2019). Weak magnetic fields alter stem cell–mediated growth. Sci Adv. 5(1): eaau7201.
分类
细胞生物学 > 组织分析 > 生理学
生物物理学 > 生物工程
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link














