- EN - English
- CN - 中文
Tetrazine Amino Acid Encoding for Rapid and Complete Protein Bioconjugation
快速且完全的蛋白质生物共轭的四嗪氨基酸编码技术
(*contributed equally to this work) 发布: 2024年08月20日第14卷第16期 DOI: 10.21769/BioProtoc.5048 浏览次数: 3181
评审: Marion HoggEdwin AntonyPallavi Prasad
Abstract
Generating protein conjugates using the bioorthogonal ligation between tetrazines and trans-cyclooctene groups avoids the need to manipulate cysteine amino acids; this ligation is rapid, site-specific, and stoichiometric and allows for labeling of proteins in complex biological environments. Here, we provide a protocol for the expression of conjugation-ready proteins at high yields in Escherichia coli with greater than 95% encoding and labeling fidelity. This protocol focuses on installing the Tet2 tetrazine amino acid using an optimized genetic code expansion (GCE) machinery system, Tet2 pAJE-E7, to direct Tet2 encoding at TAG stop codons in BL21 E. coli strains, enabling reproducible expression of Tet2-proteins that quantitatively react with trans-cyclooctene (TCO) groups within 5 min at room temperature and physiological pH. The use of the BL21 derivative B95(DE3) minimizes premature truncation byproducts caused by incomplete suppression of TAG stop codons, which makes it possible to use more diverse protein construct designs. Here, using a superfolder green fluorescent protein construct as an example protein, we describe in detail a four-day process for encoding Tet2 with yields of ~200 mg per liter of culture. Additionally, a simple and fast diagnostic gel electrophoretic mobility shift assay is described to confirm Tet2-Et encoding and reactivity. Finally, strategies are discussed to adapt the protocol to alternative proteins of interest and optimize expression yields and reactivity for that protein.
Key features
• Protocol describes site-specific encoding of the tetrazine amino acid Tet2-Et into proteins for bioorthogonal, quantitative, and rapid attachment of trans-cyclooctene-containing labels.
• Protocol uses auto-induction methods for the production Tet2-Et protein in E. coli.
• This protocol focuses on Tet-protein expressions in BL21(DE3) and B95(DE3) strains, which take approximately 4 days to complete.
• SDS-PAGE mobility shift assay using a strained TCO-PEG5000 (sTCO-PEG5000) reagent provides a simple, generalizable method for testing Tet-protein reactivity.
Keywords: 1,2,4,5-tetrazine (Tet) (1,2,4,5-四嗪(Tet))Graphical overview
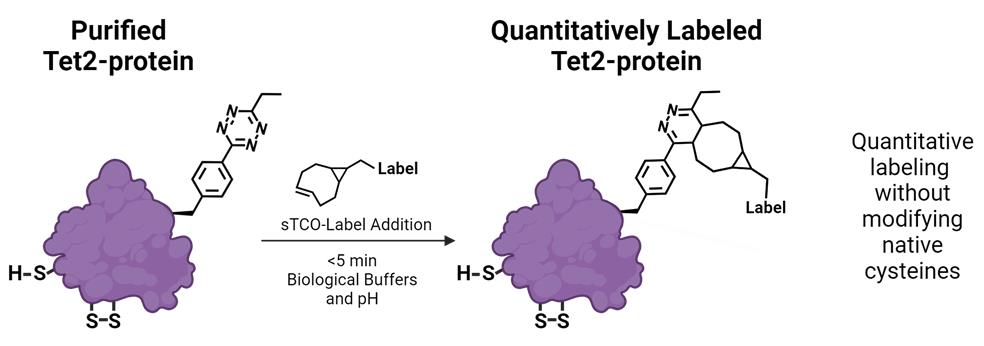
Background
The facile ability to quantitatively and rapidly attach molecules onto proteins without needing to remove or modify native cysteine residues would greatly advance the study of proteins and the development of homogeneous protein reagents, therapeutics, and diagnostics. Previously, we have demonstrated that site-specific, translational encoding of tetrazine noncanonical amino acids (Tet-ncAAs) into proteins via genetic code expansion (GCE) provides the required qualities of ideal bioorthogonal reactions to advance protein labeling [1,2]. Three of these essential qualities are (1) an exceptionally fast bioorthogonal reaction that occurs under biological conditions enabling complete labeling in short reaction times, (2) a high-fidelity GCE system that ensures that all encoding sites contain the ncAA, and (3) the ncAA is stable during encoding in biological environments so that all sites are reactive. While a variety of encodable labeling strategies are available [3,4], the encoding of Tet-ncAAs into proteins and subsequent attachment of labels containing a trans-cyclooctene (TCO) functional group stands out as the only strategy that has these three qualities for routine, quantitative protein labeling. These advantages have been leveraged to produce systems for homogeneous protein conjugation to surfaces [5,6], highly effective anti-viral nanobody conjugates [2], and the attachment of spectroscopic probes both in vitro [7] and in vivo [8].
Here, we describe the GCE encoding of an ethyl-substituted 1,2,4,5-tetrazine ncAA (Tet2-Et) and its in vitro reaction with sTCO labels having a second-order rate constant of ~104 M-1·s-1 at room temperature and physiological pH (Figure 1). We use an engineered Tet2-Et RS/tRNA pair with optimized efficiency and fidelity and plasmid construct that provides high-fidelity encoding (i.e., > 95% encoding accuracy) and quantitative labeling [1]. This protocol outlines the expression of a control protein, superfolder green fluorescent protein (sfGFP) with a TAG codon at the N150 site (sfGFP150 [9]) that yields Tet-containing sfGFP (sfGFPTet2-Et) with yields of ~200 mg per liter of culture (Figure 1, Table 1). This Tet2-Et expression system requires two plasmids: (1) the pAJE-E7 GCE machinery plasmid expressing the Tet2-Et RS/tRNA pair, and (2) a plasmid that expresses a gene of interest containing the TAG stop codon at the intended site of Tet encoding. Compatible expression hosts include the standard IPTG/lactose-inducible BL21(DE3) cell line or B95(DE3) ΔAΔfabR, which minimizes premature protein truncation at TAG-encoding sites [10]. Both strains are compatible with target protein expression from classical pET vectors (e.g., pET28). Expressions are performed in auto-induction media (AIM) for reproducible, high-yielding Tet2-protein production. Finally, we describe an electrophoresis mobility shift assay using TCO-functionalized PEG5000 for quick confirmation of accurate Tet encoding into proteins, the stability of TCO reagents, and the efficiency of protein conjugation. Completion of this protocol, including the confirmation of Tet2-Et encoding into sfGFP, takes approximately four days. Discussions on adapting this protocol for encoding Tet2-Et into biologically relevant proteins are provided.
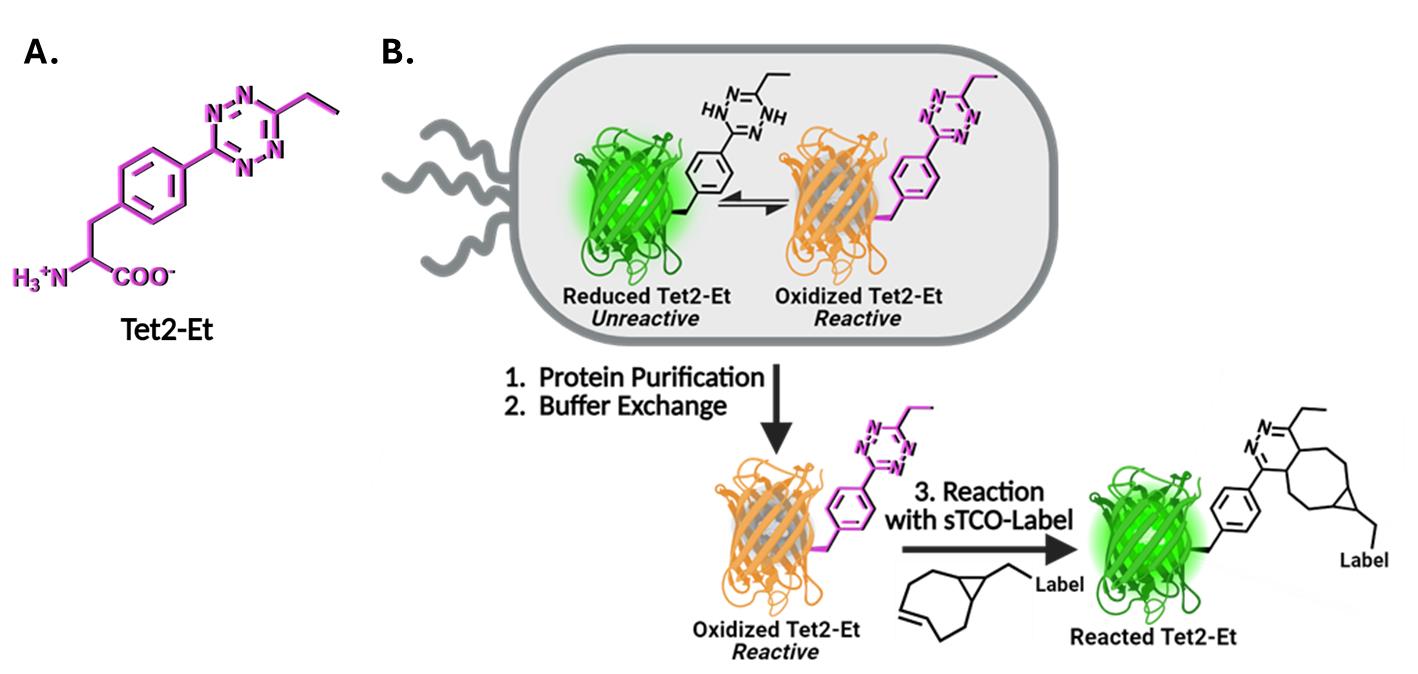
Figure 1. Genetic code expansion (GCE) encoding of tetrazine noncanonical amino acids (Tet-ncAA) and quantitative reaction with trans-cyclooctene (TCO) labels. (A) Structure of the Tet2-Et ncAA in its oxidized, reactive state. (B) During protein expression, sfGFP150 with encoded Tet2-Et exists in an equilibrium between an unreactive reduced state (green, top left) and the reactive oxidized state (orange, top right). The orange color of reactive sfGFPTet2-Et results from Tet2 quenching of sfGFP fluorescence when Tet2-Et is encoded at site N150. This fluorescence quenching does not occur when Tet2-Et is in the reduced form. Upon purification, buffer exchange, and exposure to ambient oxygen, any reduced (green) Tet2-sfGFP protein quickly oxidizes and can be quantitatively reacted with the desired sTCO labeling reagent. After reaction with sTCO, Tet2-Et no longer quenches sfGFP fluorescence. See General notes 1 and 2 for more information on Tet2 reactions and redox properties, respectively.
Materials and reagents
Biological materials
Strains
BL21(DE3) (Thermo Fisher, catalog number: EC0114). This strain of E. coli is optimized for over-expression of target proteins under the T7 transcriptional promoter that is commonly found in standard pET vectors. This strain contains a genomic copy of the T7 RNA polymerase controlled by the lacUV5 promoter so that upon introduction with IPTG, the T7 polymerase is expressed and transcribes the target gene to produce high quantities of recombinant target protein. This strain contains Release Factor 1 (RF1), the protein responsible for terminating translation at TAG amber stop codons so that when encoding Tet2-Et at TAG codons of target proteins, truncated protein will be produced along with full-length Tet2-protein. To avoid co-purification of truncated protein with full-length protein, C-terminal purification tags are recommended. For proteins that self-assemble into homo-multimers (dimers, trimers, etc.), purification can be challenging due to the possible co-purification of truncated forms that are incorporated as subunits in the assembly. If using N-terminal purification/solubilization tags, additional purification steps may be needed to remove truncated protein species. See Troubleshooting #1 for more discussion.
B95(DE3) ΔAΔfabR (Addgene, catalog number: 197655). This strain is a robustly growing BL21(DE3) derivative that lacks release factor-1 (RF1), the protein responsible for translation termination at TAG codons, as well as a spontaneously mutated fabR gene [10]. Endogenous TAG stop codons in 95 genes were mutated to TAA or TGA to maintain cellular health and minimize unwanted readthrough due to RF1 knockout. This strain is preferred over BL21(DE3) for protein expression because by lacking RF1, TAG codon suppression by GCE machinery is more efficient, and the production of truncated protein (caused by early TAG site termination) is minimized. B95(DE3) ΔAΔfabR cells require lower concentrations of antibiotics to maintain normal growth rates.
DH10B (Thermo Fisher, catalog number: EC0113). This strain can be used for faithful propagation of plasmids and for cloning needs when users clone their genes of interest into their preferred plasmid backbone. Do not use this strain for protein expression. Though we have not explicitly tested all of them, other classical cloning strains of E. coli can be used in place of DH10b, including NEB 10-beta (New England BioLabs, catalog number: C3019H), DH5α (e.g., Thermo Fisher, catalog number: 18258012), NEB 5-alpha (New England BioLabs, catalog number: C2987H), or TOP10 (Thermo Fisher, catalog number: C404010).
Plasmids
pAJE3-E7 (Addgene, catalog number: 214359). Machinery plasmid for Tet2-Et incorporation that expresses a copy of the Methanocaldococcus jannaschii (Mj)-TyrRS-(E7)-Tet2-Et RS for faithful Tet2-Et incorporation as well as a copy of its cognate amber codon suppressing tRNA under constitutive lpp promoters. This plasmid confers spectinomycin resistance and harbors a recently developed high-copy synthetic origin of replication [1,11]. This synthetic origin of replication in pAJE plasmids can be stably propagated in cells that contain other plasmids having any of the standard or typical origins of replications, such as ColE1/pBR322/pMB1, p15A, and CDF origins, adding to the utility and versatility of this machinery plasmid.
pET28-sfGFPWT (Addgene, catalog number: 85492). Expresses wild-type sfGFP control protein with C-terminal His6 tag, under a T7 transcriptional promoter, kanamycin resistance, and pBR322 origin of replication. sfGFP is expressed by the addition of IPTG or lactose.
pET28-sfGFP150 (Addgene, catalog number: 85493). Same as above except the sfGFP gene contains a TAG amber stop codon at site N150. The TAG codon is used to direct the translational encoding of Tet2-Et.
pET28-[GOI]WT (you must create). Expresses your wild-type protein of interest (POI). You can clone your POI into pET28 by removing the sfGFP gene via restriction digest with NcoI and XhoI enzymes and replacing it with your gene of interest (GOI) using standard cloning techniques (e.g., ligation, Gibson Assembly, or SLiCE [12]). For the reasons mentioned above, a C-terminal purification tag is preferred if using the BL21(DE3) strain for expression. For helpful tips on construct design, see Troubleshooting tip 1.
pET28-[GOI]TAG (you must create). Expresses your POI with Tet2-Et encoded at a TAG codon. You can clone your POI into pET28 backbone by removing the sfGFP gene by restriction digest with NcoI and XhoI and replacing it with your gene of interest (GOI) using standard cloning techniques (e.g., ligation, Gibson Assembly, or SLiCE). Using site-directed mutagenesis, change the codon to TAG (the amber stop codon) where you intend to encode Tet2-Et into your POI. See section B for recommendations on TAG site selection.
Reagents
Essential reagents
Tryptone (e.g., VWR, catalog number: 97063-386)
Yeast extract (e.g., VWR, catalog number: 97064-368)
NaCl (e.g., VWR, catalog number: 97061-274)
α-D-glucose (e.g., VWR, catalog number: 97061-168)
α-lactose (e.g., VWR, catalog number: AAA11074-0B)
Glycerol (e.g., VWR, catalog number: BDH24388.320)
Na2HPO4 (sodium phosphate dibasic) (e.g., VWR, catalog number: 97061-586)
KH2PO4 (potassium monobasic) (e.g., VWR, catalog number: BDH9268)
K2HPO4 (potassium dibasic) (e.g., VWR, catalog number: 97062-234)
NH4Cl (e.g., VWR, catalog number: 12125-02-9)
(NH4)2SO4 (e.g., VWR, catalog number: 7783-20-2)
Na2SO4 (e.g., VWR, catalog number: 7757-82-6)
MgSO4 (e.g., VWR, catalog number: 7487-88-9)
CaCl2ᐧ2H2O (e.g., VWR, catalog number: 10035-04-8)
MnCl2ᐧ4H2O (e.g., VWR, catalog number: 13446-34-9)
ZnSO4ᐧ7H2O (e.g., VWR, catalog number: 7446-20-0)
CoCl2ᐧ6H2O (e.g., VWR, catalog number: 7791-13-1)
CuCl2 (e.g., VWR, catalog number: 10125-13-0)
NiCl2 (e.g., VWR, catalog number: 7791-20-0)
Na2MoO4ᐧ2H2O (e.g., VWR, catalog number: 10102-40-6)
Na2SeO3 (e.g., VWR, catalog number: 10102-18-8)
H3BO3 (e.g., VWR, catalog number: 10043-35-3)
FeCl3 (e.g., VWR, catalog number: 7705-08-0)
L-arabinose (e.g., VWR, catalog number: 5328-37-0)
Kanamycin (e.g., VWR, catalog number: 75856-684)
Spectinomycin (e.g., VWR, catalog number: 89156-368)
Isopropyl β-D-1-thiogalactopyranoside (IPTG) (e.g., Anatrace, catalog number: I1003)
Agar (e.g., VWR, catalog number: 97064-336)
Tet2-Et amino acid (see General note 4 for reagent availability)
N,N-Dimethylformamide (DMF) (VWR, catalog number: BDH1117-4LG)
sTCO-PEG5000 (see General note 4 for reagent availability)
Sodium dodecyl sulfate (SDS) (VWR, catalog number: JT4095-4)
Silicone emulsion, Antifoam B® (VRW, catalog number: JTB531-5)
Reagents necessary only for defined auto-induction media
Glutamic acid, Na salt (e.g., VWR, catalog number: 56-86-0)
Aspartic acid (e.g., VWR, catalog number: 56-84-8)
Lysine-HCl (e.g., VWR, catalog number: 657-27-2)
Arginine-HCl (e.g., VWR, catalog number: 1119-34-2)
Histidine-HCl-H2O (e.g., VWR, catalog number: 5934-29-2)
Alanine (e.g., VWR, catalog number: 56-41-7)
Proline (e.g., VWR, catalog number: 147-85-3)
Glycine (e.g., VWR, catalog number: 56-40-6)
Threonine (e.g., VWR, catalog number: 72-19-5)
Serine (e.g., VWR, catalog number: 56-45-1)
Glutamine (e.g., VWR, catalog number: 56-85-9)
Asparagine-H2O (e.g., VWR, catalog number: 5794-13-8)
Valine (e.g., VWR, catalog number: 72-18-4)
Leucine (e.g., VWR, catalog number: 61-90-5)
Isoleucine (e.g., VWR, catalog number: 73-32-5)
Phenylalanine (e.g., VWR, catalog number: 63-91-2)
Tryptophan (e.g., VWR, catalog number: 73-22-3)
Methionine (e.g., VWR, catalog number: 63-68-3)
Solutions
Essential solutions
LB/agar media (see Recipes)
2× YT media (see Recipes)
SOC media (see Recipes)
Kanamycin stock (see Recipes)
Spectinomycin stock (see Recipes)
1 M IPTG (see Recipes)
25× M salts (see Recipes)
Trace metal stock solution (5,000×) (see Recipes)
50× 5052 solution (see Recipes)
Tet2-Et solution (see Recipes)
ZY media (see Recipes)
ZY non-induction (ZY-NIM) and auto-induction media (ZY-AIM) (see Recipes)
Solutions necessary only for defined auto-induction media
Aspartate [5% (w/v), pH 7.5] (see Recipes)
18 amino acid mix (25×) (see Recipes)
Defined-NIM and AIM media (see Recipes)
Recipes
LB/agar media (0.5 L)
Reagent Final concentration Amount Tryptone 1 % (w/v) 5 g Yeast extract 0.5 % (w/v) 2.5 g NaCl 1.0 % (w/v) 5 g Agar 1.5 % (w/v) 7.5 g H2O n/a To 500 mL Total n/a 500 mL After mixing reagents thoroughly, autoclave on standard liquid setting to sterilize. Note the agar will not go into solution until autoclaved.
After autoclaving, gently swirl the bottle to ensure molten agar is evenly mixed.
Notes:
i. Store LB/agar bottle in a 55 °C oven and pour plates on an as-needed basis. LB/agar can be stored in molten form for ~2 weeks if sterility is maintained.
ii. If an oven is not available, plates can be poured with antibiotics once LB/agar is sufficiently cooled to touch. Plates can be stored at 4 °C for up to a week.
2× YT media (1 L)
Reagent Final concentration Amount Tryptone 1.6 % (w/v) 16 g Yeast extract 1.0 % (w/v) 10 g NaCl 0.5 % (w/v) 5 g H2O n/a To 1000 mL Total n/a 1 L After mixing reagents thoroughly, autoclave on the standard liquid setting to sterilize.
After autoclaving, allow it to cool to room temperature before use.
SOC media (50 mL)
Reagent Final concentration Amount 2× YT media n/a 49 mL 1 M MgSO4 10 mM 0.5 mL 40% (w/v) α-D-glucose 0.4% (w/v) or ~20 mM 0.5 mL Total n/a 50 mL 1 M MgSO4 can be made by mixing 12.3 g of MgSO4·7H2O in water up to 50 mL total volume. Adjust mass of MgSO4 accordingly if using a salt with a different hydration status.
40% (w/v) α-D-glucose can be made by mixing 20 g of α-D-glucose with water up to 50 mL total volume. Mix thoroughly until glucose is dissolved. Gentle heating in a microwave may facilitate the dissolution of glucose.
Sterilize MgSO4, glucose, and 2× YT solutions individually by autoclaving. Allow each component to cool to room temperature and mix as indicated above. Maintain sterility while adding components together.
It is easy to contaminate SOC. We suggest breaking this into 5 × 10 mL aliquots before use or making smaller batches. If sterility is maintained, SOC can be stored at room temperature indefinitely. It can also be stored at -20 °C but avoid repeated freeze/thaws.
Kanamycin stock (10 mL)
Reagent Final concentration Amount Kanamycin 50 mg/mL 0.5 g H2O n/a To 10 mL Total n/a 10 mL Sterilize by filtering with a 0.2 μm syringe-end filter.
Store in 1 mL aliquots at -20 °C.
Spectinomycin stock (10 mL)
Reagent Final concentration Amount Spectinomycin 100 mg/mL 1 g H2O n/a To 10 mL Total n/a 10 mL Sterilize by filtering with a 0.2 μm syringe-end filter.
Store in 1 mL aliquots at -20 °C.
Note: Do not confuse spectinomycin with streptomycin. These antibiotics are not interchangeable.
1 M IPTG (10 mL)
Reagent Final concentration Amount IPTG 1 M 2.3 g H2O n/a To 10 mL Total n/a 10 mL Sterilize by filtering with a 0.2 μm syringe-end filter.
Store in 1 mL aliquots at -20 °C.
25× M salts
Reagent 25× concentration Amount for 25× Na2HPO4 0.625 M 88.7 g KH2PO4 0.625 M 85.1 g NH4Cl 1.25 M 66.9 g Na2SO4 0.125 M 17.8 g H2O n/a to 1 L Total 1 L Add the above components to a 2 L beaker containing a magnetic stir bar. Add water up to 900 mL and mix until all components have dissolved. Add the remaining volume of water to reach 1 L.
Weights indicated are based on anhydrous salts. If using hydrated phosphate salts, adjust the weights accordingly to maintain indicated molarities.
Trace metal stock solution (5,000×)
Reagent Concentration Amount for individual 30 mL stocks 5,000× 1× CaCl2·2H2O 20 mM 4 µM 8.82 g MnCl2·4H2O 10 mM 2 µM 5.93 g ZnSO4·7H2O 2 M 2 µM 8.62 g CoCl2·6H2O 2 mM 0.4 µM 1.32 g CuCl2 2 mM 0.4 µM 807 mg NiCl2 2 mM 0.4 µM 777 mg Na2SeO3 2 mM 0.4 µM 1.03 g Na2MoO4·2H2O 2 mM 0.4 µM 1.45 g H3BO3 2 mM 0.4 µM 371 mg FeCl3 50 mM 10 µM 486 mg H2O n/a To 30 mL For each of the metals above (except FeCl3), make individual stock solutions using the indicated masses and dissolve in Milli-Q water up to 30 mL of total volume. Autoclave each metal solution separately to sterilize. The FeCl3 must be dissolved in 0.1 M HCl up to 30 mL of total volume and then filtered (through a 0.2 µm filter) to remove insoluble material and sterilize (do not autoclave).
Once all individual stock solutions are prepared, add 500 µL of each stock solution (except FeCl3) to 20.5 mL of sterile Milli-Q water. Then, add 25 mL of the FeCl3 solution. The total volume should be exactly 50 mL.
This stock solution might show minor precipitation over time but is stable at 15–25 °C for years.
50× 5052 solution (500 mL)
Reagent Final concentration Amount α-D-glucose 25 mg/mL 12.5 g α-lactose 100 mg/mL 50 g Glycerol 25% (v/v) 125 mL H2O n/a to 500 mL Total n/a 500 mL Add the glucose, lactose, and glycerol components to roughly 300 mL of warm water in a 0.5 L beaker containing a magnetic stir bar. Mix until all solutions have dissolved. Additional heating may be required via microwave to encourage lactose dissolution (CAUTION: Remove magnetic stir bar before microwaving). Once fully dissolved, add the remaining volume of water to reach 500 mL.
Autoclave on liquid cycle to sterilize.
Tet2-Et solution
Reagent Final concentration Amount Tet2-Et 100 mM 8.5 mg DMF n/a to 275 µL Total n/a 275 µL Vortex solution after combining to ensure all Tet2-Et has dissolved.
Solution can be stored at -20 °C for months but may come out of solution and require additional vortexing upon freeze/thaw cycles. For optimal expressions, prepare the solution directly before use to avoid freeze/thaw cycles.
ZY media
Reagent Final concentration Amount Tryptone 1% (w/v) 10 g Yeast extract 0.5% (w/v) 5 g H2O n/a to 1 L Total n/a 1 L Add the above components to a 1 L beaker containing a magnetic stir bar. Add water up to 900 mL and mix until all solutions have dissolved. Add the remaining volume of water to reach 1 L.
Autoclave for sterilization.
ZY non-induction (ZY-NIM) and auto-induction media (ZY-AIM)
Reagent ZY-NIM ZY-AIM Amount Amount ZY media 47 mL 47 mL MgSO4 0.1 mL 0.1 mL 25× M salts 2 mL 2 mL 50× 5052 - 1 mL 40% (w/v) α-D-glucose 0.625 mL - Trace metal (5,000×) 10 µL 10 µL Total 50 mL 50 mL When preparing media, dilute the concentrated components into ZY media. Do not mix concentrated stocks and then dilute with ZY media.
For BL21(DE3), the final concentration for spectinomycin and kanamycin should be 100 µg/mL and 50 µg/mL, respectively. For B95(DE3) expressions, the final concentration for spectinomycin and kanamycin should be 50 µg/mL and 25 µg/mL, respectively.
Prepare immediately before use with a sterile technique.
Aspartate [5% (w/v), pH 7.5]
Reagent Final concentration Amount Aspartate 5% (w/v) 50 g H2O n/a To 1 L Total n/a 1 L Mix by placing a suitable magnetic stir bar in a 2 L beaker and add 900 mL of water to the graduated cylinder. While stirring, add the appropriate amount of L-aspartic acid and adjust pH to 7.5 with 8 M NaOH. Add the remaining volume of H2O to bring the solution to the final volume of 1 L.
Sterilize by autoclaving on liquid setting.
18 amino acid mix (25×) (1 L)
Reagent Concentration Amount for 25× 25× 1× Glutamic acid, Na salt 200 µg/mL 8 µg/mL 5 g Aspartic acid 200 µg/mL 8 µg/mL 5 g Lysine-HCl 200 µg/mL 8 µg/mL 5 g Arginine-HCl 200 µg/mL 8 µg/mL 5 g Histidine-HCl-H2O 200 µg/mL 8 µg/mL 5 g Alanine 200 µg/mL 8 µg/mL 5 g Proline 200 µg/mL 8 µg/mL 5 g Glycine 200 µg/mL 8 µg/mL 5 g Threonine 200 µg/mL 8 µg/mL 5 g Serine 200 µg/mL 8 µg/mL 5 g Glutamine 200 µg/mL 8 µg/mL 5 g Asparagine-H2O 200 µg/mL 8 µg/mL 5 g Valine 200 µg/mL 8 µg/mL 5 g Leucine 200 µg/mL 8 µg/mL 5 g Isoleucine 200 µg/mL 8 µg/mL 5 g Phenylalanine 200 µg/mL 8 µg/mL 5 g Tryptophan 200 µg/mL 8 µg/mL 5 g Methionine 200 µg/mL 8 µg/mL 5 g H2O n/a n/a To 1 L Total n/a n/a 1 L Add 800 mL of water to a 1 L beaker, then add 5 g of each amino acid while stirring with a magnetic stir bar. Since some amino acids have trouble dissolving in solution, warming the water prior to adding the amino acids can aid in the dissolution process. It may take several hours for each component to fully dissolve. Finally, bring the volume to 1 L with water.
Sterilize by filtration.
Aliquot 45 mL of 25× 18-amino acid mix into sterile 50 mL conicals.
Store aliquots at -20 °C. Thaw working aliquot as needed, which can be stored stably at 4 °C for several months provided sterility is maintained.
Defined-NIM and AIM media
Reagent NIM AIM Amount Amount Aspartate [5% (w/v) pH 7.5] 2.5 mL 2.5 mL 50× 5052 - 1 mL 18 amino acid mix 2.0 mL 2.0 mL 25× M salts 2.0 mL 2.0 mL MgSO4 (1 M) 100 µL 100 µL Glucose [40% (w/v)] 6.25 mL - Trace metal solution (5,000×) 10 µL 10 µL Sterile H2O 36.64 mL 41.89 mL Total 50 mL 50 mL When preparing media, add the concentrated components to sterile H2O, do not mix concentrated stocks, and then dilute with sterile H2O.
For BL21(DE3), the final concentration for spectinomycin and kanamycin should be 100 µg/mL and 50 µg/mL, respectively. For B95, the final concentration for spectinomycin and kanamycin should be 50 µg/mL and 25 µg/mL, respectively.
Prepare immediately before use with a sterile technique.
Laboratory supplies
1.7 mL Eppendorf tubes (e.g., VWR, catalog number: 87003-294)
100 mm plates (e.g., VWR, catalog number: 470210-568)
500 mL graduated cylinder (e.g., VWR, catalog number: 470344-338)
15 mL conical tubes (e.g., VWR, catalog number: 89126-798)
50 mL conical tubes (e.g., VWR, catalog number: 89039-656)
14 mL sterile culture tubes (e.g., VWR, catalog number: 60818-689)
250 mL baffled flasks (e.g., VWR, catalog number: 89095-266)
Micro pipette tips 10 µL (e.g., VWR, catalog number: 76323-394)
Micro pipette tips 200 µL (e.g., VWR, catalog number: 76323-390)
Micro pipette tips 1,000 µL (e.g., VWR, catalog number: 76323-454)
12% Mini-PROTEAN® TGXTM precast protein gels, 15 well, 15 µL (Bio-Rad, catalog number: 4561046)
Mini-PROTEAN® Tetra companion running module (Bio-Rad, catalog number: 1658038)
Mini-PROTEAN® Tetra vertical electrophoresis cell for mini precast gels, 4-gel (Bio-Rad, catalog number: 1658004)
Disposable PD-10 desalting column, with Sephadex G-25 resin, 1.0–2.5 mL samples (Cytiva, catalog number: 17085101)
TALON® SuperflowTM (VWR, catalog number: CA71006-006)
Nalgene® bottle-top sterile filter (Millipore Sigma, catalog number: Z358223-12EA)
Equipment
Autoclave capable of sterilizing liquid media and culturing materials at 121 °C and of a saturated steam pressure of 15 PSI
Expression equipment:
Static incubator for growing LB/agar plates (set to 37 °C) (e.g., VWR, catalog number: 97025-630)
Shaker incubator for growing liquid cultures (e.g., New Brunswick I26R, Eppendorf, catalog number: M1324-0004)
i. Shaker should be able to rotate at 200–250 rpm.
ii. Refrigeration is necessary for expressions below room temperature (< 25 °C).
iii. Shaker deck should have clamps to hold 250 mL and 2.8 L Fernbach flasks.
Optical density 600 nm spectrophotometer (e.g., Ultrospec 10, Biochrome, catalog number: 80-2116-30)
Fluorometer capable of reading sfGFP fluorescence (excitation 488 nm/emission 512 nm). Handheld fluorometers work well for routine fluorescence reads (e.g., PicoFluor from Turner Biosystems)
Freezer (-20 °C) for storing plasmids and antibiotics (e.g., Fisher Scientific, catalog number: 10-549-264)
Ice machine (e.g., Fisher Scientific, catalog number: 09-540-003)
Software and datasets
ImageJ
Procedure
文章信息
稿件历史记录
提交日期: Apr 20, 2024
接收日期: Jul 7, 2024
在线发布日期: Jul 25, 2024
出版日期: Aug 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Eddins, A. J., Pung, A. H., Cooley, R. B. and Mehl, R. A. (2024). Tetrazine Amino Acid Encoding for Rapid and Complete Protein Bioconjugation. Bio-protocol 14(16): e5048. DOI: 10.21769/BioProtoc.5048.
分类
生物工程 > 合成生物学 > 基因修饰
生物化学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












