- EN - English
- CN - 中文
Approach for Electrophysiological Studies of Spinal Lamina X Neurons
脊髓板层X神经元电生理研究方法
发布: 2024年07月20日第14卷第14期 DOI: 10.21769/BioProtoc.5035 浏览次数: 2133
评审: Sarajo MohantaMayank GautamAnonymous reviewer(s)
Abstract
Despite playing diverse physiological roles, the area surrounding the central canal, lamina X, remains one of the least studied spinal cord regions. Technical challenges and limitations of the commonly used experimental approaches are the main difficulties that hamper lamina X research. In the current protocol, we describe a reliable method for functional investigation of lamina X neurons that requires neither time-consuming slicing nor sophisticated in vivo experiments. Our approach relies on ex vivo hemisected spinal cord preparation that preserves the rostrocaudal and mediolateral spinal architecture as well as the dorsal roots, and infrared LED oblique illumination for visually guided patch clamp in thick blocks of tissue. When coupled with electric stimulation of the spared dorsal roots, electrophysiological recordings provide information on primary afferent inputs to lamina X neurons from myelinated and non-myelinated fibers and allow estimating primary afferent–driven presynaptic inhibition. Overall, we describe a simple, time-efficient, inexpensive, and versatile approach for lamina X research.
Key features
• Quick and easy preparation procedure that grants access to lamina X neurons without spinal cord slicing
• Preserved rostrocaudal and mediolateral connectivity and preserved primary afferent supply
• Ability to perform electrophysiological recordings in combination with dorsal root stimulations allowing to study afferent inputs and presynaptic inhibition of lamina X neurons
Keywords: Spinal cord (脊髓)Graphical overview
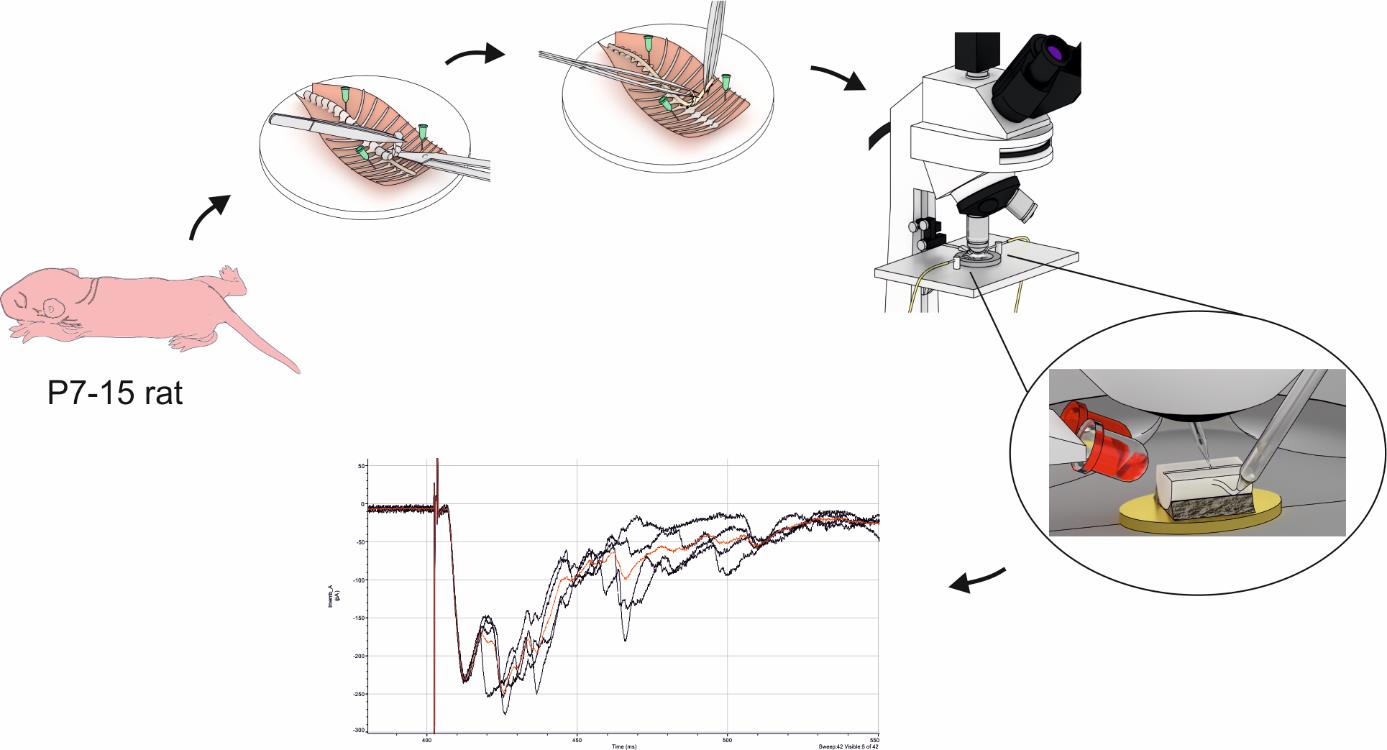
Preparation of ex vivo hemisected spinal cord and electrophysiological recordings from lamina X neurons
Background
Based on its cytoarchitectonics, the spinal cord is subdivided into ten regions called laminae [1]. Spinal laminae are studied to a different extent: while superficial ones have been explored in much detail, our knowledge about the deeper laminae is unsatisfactory. In particular, lamina X surrounding the central canal remains one of the least studied spinal regions despite playing diverse physiological roles. Evidence indicates that lamina X is crucial for visceral nociception [2–6] and participates in somatosensory processing [4,7,8], locomotion [9], and autonomic regulation [10]. Yet, reports on lamina X are scarce and derive mostly from morphological and immunohistochemical data. Functional investigations of lamina X are even rarer given that they mainly rely on two approaches. The first one involves in vivo electrophysiological recordings using either an extracellular electrode [4,7] or a blind patch clamp technique [10,11]. Both methods require post hoc tissue analysis to confirm recordings from lamina X cells. The other approach utilizes visually guided patch clamp in spinal cord slices [13–23] in which intrinsic spinal cord connectivity and primary afferent inputs are disrupted. Both mentioned methods are technically challenging and have inherent flaws that limit the functional investigation of lamina X. Thus, an experimental approach circumventing these limitations would greatly boost the lamina X research.
Here, we provide a detailed protocol for electrophysiological studies of lamina X neurons. The described procedure is easy and reliable. It allows recording over prolonged periods (up to 6–8 h) and requires only very basic equipment for ex vivo electrophysiology. The main advantage of our approach is the use of a spinal cord hemisected at the sagittal midline, which fully preserves rostrocaudal and mediolateral spinal architecture and spares the dorsal roots. Unlike spinal cord slices, the hemisected preparation is produced without any specialized device and needs little to no incubation; the preparation is ready for use within half an hour after decapitation. For cell visualization, we use infrared LED oblique illumination, which is specifically designed to work with thick blocks of tissue [24,25]. A combination of hemisected spinal cord preparation together with oblique LED illumination gives a unique opportunity to perform visually guided patch clamp in practically intact tissue. This is particularly beneficial for studying primary afferent inputs to lamina X and their modulation. Patch clamp experiments coupled with electrical dorsal root stimulation via suction electrodes enable recording elicited postsynaptic currents in lamina X neurons. Different parameters of electrical stimuli allow the examination of direct synaptic inputs from thinly myelinated A fibers and non-myelinated C fibers as well as their primary afferent–driven presynaptic inhibition. The current protocol is suitable for using both young rats and mice of various genetic backgrounds. Additionally, the protocol may be applied for studying various cell populations (such as spinothalamic, spinohypothalamic, and sympathetic preganglionic neurons) preliminary labeled with retrograde dyes [26]. Finally, the preserved rostrocaudal architecture of the hemisected preparation allows complementing the protocol with the stimulation of descending (top-down) fibers of the spinal cord [27].
Materials and reagents
Biological materials
Wistar rats P7–14 (Charles River, strain code: 003)
Sucrose (Sigma-Aldrich, catalog number: S0389)
Glucose (Sigma-Aldrich, catalog number: G8270)
Sodium chloride (NaCl) (Sigma-Aldrich, catalog number: S9888)
Sodium bicarbonate (NaHCO3) (Sigma-Aldrich, catalog number: S0751)
Sodium monophosphate (NaH2PO4) (Sigma-Aldrich, catalog number: S6040)
Potassium chloride (KCl) (Sigma-Aldrich, catalog number: P9333)
Magnesium chloride (MgCl2·6H2O) (Sigma-Aldrich, catalog number: M2670)
Calcium chloride (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C7902)
Potassium gluconate (Sigma-Aldrich, catalog number: G4500)
Sodium ATP (Na2ATP) (Sigma-Aldrich, catalog number: A3377)
Sodium GTP (NaGTP) (Sigma-Aldrich, catalog number: G8877)
HEPES (Sigma-Aldrich, catalog number: H3375)
EGTA (Sigma-Aldrich, catalog number: E0396)
Sodium ascorbate (Sigma-Aldrich, catalog number: 11140)
Sodium pyruvate (Sigma-Aldrich, catalog number: P2256)
Isoflurane (Abbvie, catalog number: B506)
95% O2 and 5% CO2 gas mixture
Solutions
Sucrose dissection solution (see Recipes)
Krebs bicarbonate solution (see Recipes)
Potassium gluconate intracellular solution (see Recipes)
Recipes
Sucrose dissection solution (final volume 0.5 L, in ddH2O)
Reagent Final concentration Quantity Sucrose 200 mM 34,230 mg Glucose 11 mM 991 mg NaHCO3 26 mM 1092 mg NaH2PO4 1.2 mM 72 mg KCl 2 mM 74.5 mg MgCl2·6H2O 7 mM 712 mg CaCl2·2H2O 0.5 mM 37 mg Sodium ascorbate (optional) 5 mM 495 mg Sodium pyruvate (optional) 3 mM 168 mg pH 7.3–7.4 when bubbled with 95% O2 and 5% CO2; osmolarity 310–320 mOsm/kg. This solution may be used for up to two weeks after preparation if kept at 4–8 °C.
Krebs bicarbonate recording solution (final volume 0.5 L, in ddH2O)
Critical: To avoid precipitation, the solution must be bubbled with 95% O2 and 5% CO2 gas mixture before adding calcium chloride.
Reagent Final concentration Quantity or Volume NaCl 125 mM 3,653 mg Glucose 10 mM 901 mg NaHCO3 26 mM 1092 mg NaH2PO4 1.25 mM 75 mg KCl 2.5 mM 93 mg MgCl2·6H2O 1 mM 102 mg CaCl2·2H2O 2 mM 147 mg pH 7.3–7.4 when bubbled with 95% O2 and 5% CO2; osmolarity 300–310 mOsm/kg. This solution may be used for up to two weeks after preparation if kept at 4–8 °C.
Potassium gluconate intracellular solution (final volume 50 mL, in ddH2O)
Reagent Final concentration Quantity or Volume Potassium gluconate 145 mM 1,698 mg MgCl2·6H2O 2.5 mM 25.5 mg Sodium ATP 2 mM 55 mg Sodium GTP 0.5 mM 13 mg HEPES 10 mM 119 mg EGTA 0.5 mM 9.5 mg pH 7.3 adjusted with KOH; osmolarity 280–290 mOsm/kg. Make 1 mL aliquots and freeze them at -20 °C.
Laboratory supplies
Dissection dish with Sylgard-lined bottom (Living Systems Instrumentation, catalog number: DD-90-S-BLK)
25 G hypodermic needles (BD, catalog number: 300400)
30 G hypodermic needles (BD, catalog number: 304000)
Super glue (cyanoacrylate, water-resistant gel)
Glass capillaries with filament O.D. 1.5 mm, I.D. 0.86 mm (e.g., Sutter Instruments, catalog number: BF-150-86-10; Harvard Apparatus, catalog number: GC150F-10)
Thin-walled glass capillaries without filament O.D. 1.5 mm, I.D. 1.17 mm (Warner Instruments, catalog number: G150T-3)
1 mL Luer-Lok syringes (BD, catalog number: 309628)
2.5 mL Luer-Lok syringes (BD, catalog number: 300185)
Silicon tubing (VWR, catalog number: 228-0701)
Three-way tap (BD Connecta, catalog number: 394601)
Patch pipette fillers (WPI, catalog number: MF28G67-5)
Sterile syringe filters 0.22 μm (Thermo Scientific, catalog number: 171-0020)
Equipment
Dissection instruments
Dissection pad (could be made from Styrofoam tightly wrapped in foil)
Anesthesia induction chamber (VetEquip, US) or any other available
Big scissors (F.S.T., catalog number 14000-20)
Small scissors (F.S.T., catalog number 14058-11)
Coarse forceps (F.S.T., catalog number 11651-10)
Big spring scissors (F.S.T., catalog number 15025-10)
Small spring scissors (F.S.T., catalog number 15000-12)
Two curved forceps (F.S.T., catalog number 11063-07)
Small metal plate (preferably gold, should fit in experimental chamber)
Stereomicroscope (Olympus, model: SZX7)
Light source (AmScope, model: 6 W LED Dual Gooseneck Illuminator)
Upright microscope (Olympus, model: BX50WI)
60× water immersion objective (Olympus, model: LUMPlanFl 60×/0.90 W)
Low magnification (4–5×) objective (Carl Zeiss, model: Epiplan; Olympus, model Plan N)
Eyepiece graticule (any fitting the microscope)
Infrared-sensitive camera (Olympus, model: OLY-150IR or Dage-MTI, model: IR-2000)
Narrow beam (± 3°) infrared light emitting diode (Osram, models: SFH4550, SFH4545)
White light emitting diode (Dialight, model: 5219901802F)
Power supply unit (AimTTI, model: QL355T)
Gravity-fed perfusion system (any self-made or commercially available)
Peristaltic pump (Gilson, model: Minipuls 3)
Recording chamber (any fitting the microscope)
Patch clamp amplifier with respective headstage (Molecular Devices, models: Multiclamp 700B, Axopatch 200B)
Digitizer (Molecular Devices, models: Digidata 1440, Digidata 1550)
PC computer with Windows operating system
Patch clamp micromanipulator (e.g., Scientifica, model: PatchStar; Sutter Instruments, model: MP-225)
Constant current stimulator (e.g., A.M.P.I., model: ISO-FLEX; A-M Systems, model: 2100; Digitimer, model: DS3)
Vibration isolation table (CleanBench, model: TMC)
Faraday cage (Sutter Instruments, model: AT-36FC)
Small three-axis micromanipulators (Narishige, model: UN-3C)
Pipette holders (Molecular devices, model: 1-HL-U)
Pipette puller (Sutter Instruments, models: P-87, P-97)
Silver wire for electrodes (WPI, product number: AGW1510)
Ag/AgCl pellets (WPI, product number: EP1)
BNC cables
Bunsen burner
Software and datasets
pClamp (version 10.7, Molecular Devices, July 2016)
Clampfit (version 10.7, Molecular Devices, July 2016)
Origin (version 2022, OriginLab, June 2022)
MiniAnalysis (version 6.0.7, Synaptosoft Inc.)
Procedure
文章信息
稿件历史记录
提交日期: Apr 15, 2024
接收日期: Jun 16, 2024
在线发布日期: Jul 17, 2024
出版日期: Jul 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Krotov, V., Belan, P. and Voitenko, N. (2024). Approach for Electrophysiological Studies of Spinal Lamina X Neurons. Bio-protocol 14(14): e5035. DOI: 10.21769/BioProtoc.5035.
分类
生物物理学 > 电生理 > 膜片钳技术
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













