- EN - English
- CN - 中文
Characterizing ER Retention Defects of PDZ Binding Deficient Cx36 Mutants Using Confocal Microscopy
用共聚焦显微镜表征PDZ结合缺陷的Cx36突变体内质网滞留缺陷
发布: 2024年07月20日第14卷第14期 DOI: 10.21769/BioProtoc.5034 浏览次数: 1199
评审: Philipp WörsdörferAnonymous reviewer(s)
Abstract
Overexpression of proteins in transiently transfected cells is a simple way to study basic transport mechanisms and the underlying protein–protein interactions. While expression systems have obvious drawbacks compared to in vivo experiments, they allow a quick assessment of more conserved functions, for instance, ER export or sorting of proteins in the Golgi. In a previous study, our group described the formation of ER-derived removal vesicles for the gap junction protein Cx36 in transfected HEK293T cells. These removal vesicles, termed “whorls” because of their concentric structure, were formed by Cx36 channels that failed to escape the ER. In this article, we describe an imaging protocol that can be used to determine these ER retention defects for Cx36 expressed in cultured cells. The protocol we provide here employs regular confocal microscopy, which allows for sufficient resolution to reveal the characteristic shape of ER whorls.
Keywords: Connexin 36 (连接蛋白36)Background
Gap junctions are clusters of intercellular channels that directly connect the cytoplasm of adjacent cells, providing a conduit for the exchange of metabolites and ions. In chordates, gap junctions are formed by connexins, a diverse family of membrane proteins that have the ability to oligomerize into dodecameric channels. Mutations in connexin genes are the cause of a variety of inherited diseases. In many cases, these pathologies have been linked to trafficking defects that compromise the ability of the channel to form functional gap junctions [1,2]. Among the 21 connexins that have been identified in humans, only a few variants have been studied in terms of trafficking through intracellular compartments [2–4]. To study basic transport mechanisms of gap junction proteins, researchers take advantage of expression systems, such as HeLa or HEK293T cells, and transfect these cells with recombinant expression vectors. In this article, we describe a detailed protocol that can be used to determine endoplasmic reticulum (ER) retention defects for connexin 36 (Cx36). In a previous study, we described a transport defect that prevented the functional ER export of Cx36, causing the connexin to accumulate in the ER [5]. This retention mechanism promoted the formation of gap junction-like aggregates that reshaped the ER into concentric multi-membrane vesicles (Figures 1B and D), which we termed connexin whorls. These structures were characterized by several distinct features: 1) Each sheet within the whorl exhibited ultrastructural features that were indistinguishable from an actual gap junction; 2) whorls are hollow inside and their diameter varied, ranging from 0.3 to 3 µm; 3) whorls colocalized with ER-phagy receptors Tex264 (Testis-expressed protein 264) (Figure 1C) and p62; 4) whorl formation requires docking interactions of extracellular loops in Cx36 facing the ER lumen. Substituting the extracellular loop cysteines (C55 or C62) via site-directed mutagenesis prevents whorl formation. Similar whorl-like structures were reported for the lens connexin Cx50 in a previous study by Lichtenstein et al., [6], which suggests that ER-derived whorls are formed by many connexins that oligomerize in ER. Therefore, the protocol we describe here is not only applicable for Cx36 but might be used for other connexin isoforms. However, two important aspects have to be considered: 1) Some connexins, for instance Cx43, oligomerize in the Golgi [7], which would make the docking of Cx43 containing connexons in the ER impossible; and 2) whorls have to be tested for the presence of ER proteins, for instance Tex264, to determine the compartment they originated in (Figure 1C).
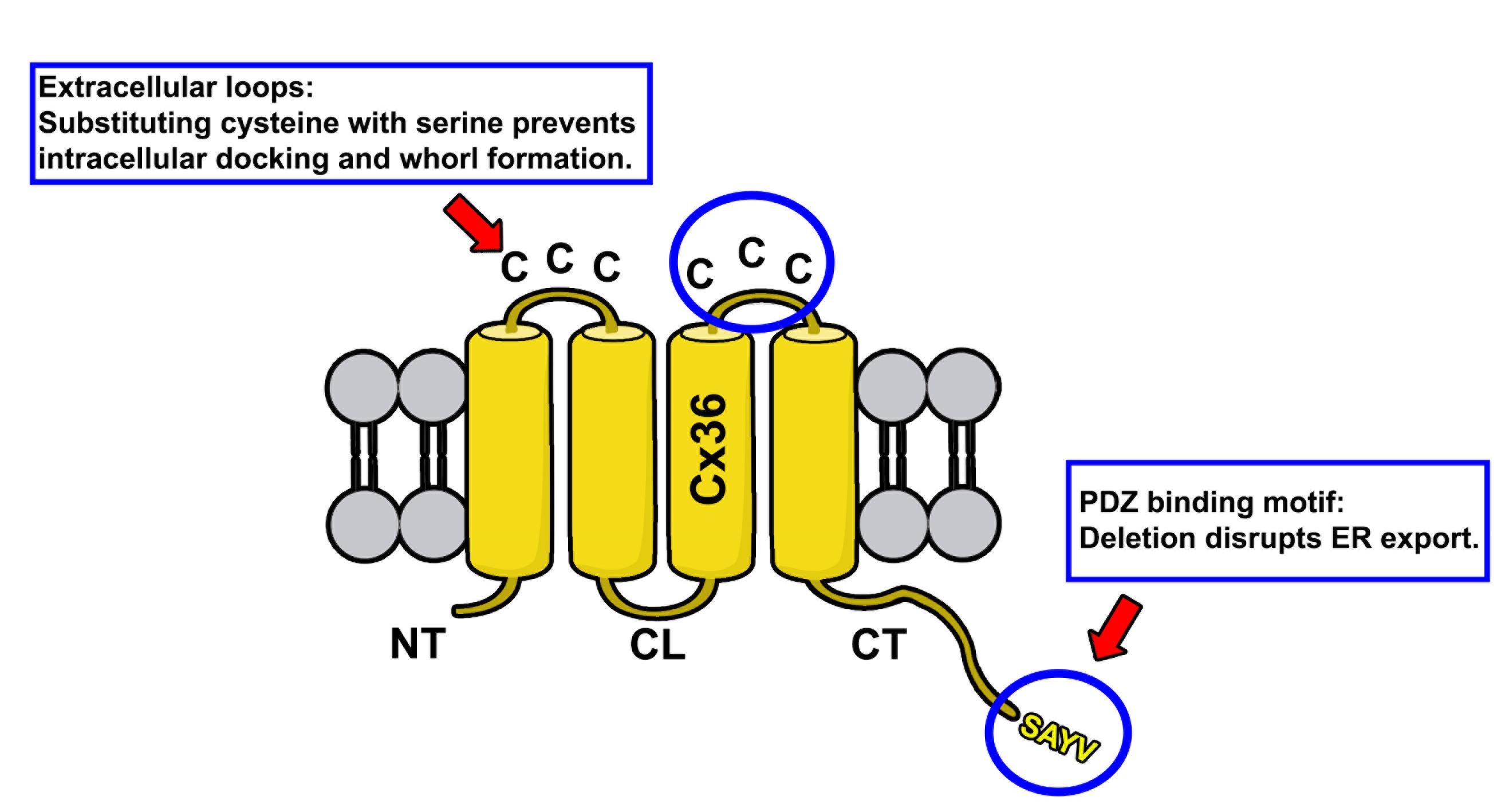
Figure 1. Membrane topology of Cx36. Protein structure of Cx36 is illustrated. The two extracellular loops of Cx36 (indicated by upper circle and red arrow) contain three cysteines. The C-terminal tail contains the PDZ binding motif consisting of the following amino acid sequence: SAYV.
Rationale for experimental design
The rationale for the design of connexin mutants we have described in Tetenborg et al., (2023) was based on the observation that truncation of the C-terminal tip in Cx36 prevents functional ER export leading to the intracellular formation of connexin whorls. To test if these multimembrane vesicles are formed by a gap junction-like docking mechanism of opposing hemichannels in the ER, we substituted cysteines in the extracellular loop of Cx36 with serines via site-directed mutagenesis. These mutations were previously shown to block gap junction formation [8] and served as a control experiment in our study to confirm that ER retention causes opposing Cx36 channels to dock intralumenally via the extracellular loops.
Materials and reagents
Biological materials
Anti Cx35/Cx36 antibody (depends on the connexin that is studied); our study focused on Cx36 (Sigma-Aldrich, catalog number: MAB3045)
Anti Testis expressed protein 264 (Tex264) (Novus Biologicals, catalog number: NBP1-89886)
Anti Golgin97 (Thermo Fisher Scientific, catalog number: PA5-30048)
Human embryonic kidney 293T cells T/17 (ATCC, catalog number: CRL-11268)
Connexin 36 expression vector (or any other connexin), map shown in Figure 1
Normal donkey serum [9] (Jackson Immunoresearch, catalog number: 017-000-121)
Donkey anti-mouse Cy3 (Jackson Immunoresearch, catalog number:715-165-160)
Donkey anti-rabbit Alexa Fluor 488 (Jackson Immunoresearch, catalog number:715-545-152)
Dulbecco’s modified Eagle’s serum (DMEM) (Thermofisher, catalog number: 12800017)
Fetal bovine serum (FBS) (Thermofisher, catalog number: A5209401)
2.5% Trypsin 10× (Thermofisher, catalog number: 15090-046)
Geneporter 2 (Amsbio, catalog number: T20200)
Paraformaldehyde (PFA) 20% (Electron Microscopy Sciences, catalog number: 15712)
Poly-l-lysine (PLL) 0.01% (Millipore, catalog number: A-005-C)
Triton-X 100 (Fisher Scientific, catalog number: BP151-100)
50 mL Falcon tube (Falcon, catalog number: 352070)
Phosphate buffered saline (PBS) (Sigma-Aldrich, catalog number: P3813)
Vectashield PLUS mounting media, DAPI (Vector Laboratories, catalog number: H-2000)
Tissue Path SuperfrostTM Plus gold (Thermofisher, catalog number: 1518846)
Normal donkey serum (NDS) (Jackson Immunoresearch, catalog number: 017-000-121)
75 cm2 cell culture flask (Corning, catalog number: 430725U)
35 mm × 10 mm style cell culture dish (Corning, catalog number: 430165)
Coverslips (Fisher Scientific, catalog number: 12541000)
24-well plate (Corning Incorporated, catalog number: 3526)
AxygenTM Maxyclear Snaplock microtubes, 1.5 mL (Fisher Scientific, catalog number: 14-222-158)
Nail polish, L.A colors, rapid dry (Electron Microscopy Sciences, catalog number: 72180)
Software and datasets
Fiji, open source [10]
Equipment
Biological safety cabinet, 1300 Series A2 (Thermofisher, catalog number: 72180)
Incubator (Eppendorf, model: Galaxy 170 S)
Eppendorf research plus pipette (Eppendorf, catalog number: 3123000012)
Confocal laser scanning microscope (Zeiss, model: LSM800)
Procedure
文章信息
稿件历史记录
提交日期: Feb 7, 2024
接收日期: Jun 11, 2024
在线发布日期: Jul 2, 2024
出版日期: Jul 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Tetenborg, S., Martinez-Soler, E. and O`Brien, J. (2024). Characterizing ER Retention Defects of PDZ Binding Deficient Cx36 Mutants Using Confocal Microscopy. Bio-protocol 14(14): e5034. DOI: 10.21769/BioProtoc.5034.
- Tetenborg, S., Liss, V., Breitsprecher, L., Timonina, K., Kotova, A., Acevedo Harnecker, A. J., Yuan, C., Shihabeddin, E., Ariakia, F., Qin, G., et al. (2023). Intralumenal docking of connexin 36 channels in the ER isolates mistrafficked protein. J Biol Chem. 299(11): 105282. https://doi.org/10.1016/j.jbc.2023.105282
分类
细胞生物学 > 细胞成像 > 荧光
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link