- EN - English
- CN - 中文
Quantification of Autophagosomes in Human Fibroblasts Using Cyto-ID® Staining and Cytation Imaging
利用Cyto-ID®染色和Cytation成像技术定量分析人类成纤维细胞中的自噬小体
发布: 2024年07月05日第14卷第13期 DOI: 10.21769/BioProtoc.5025 浏览次数: 1637
Abstract
As an essential process for the maintenance of cellular homeostasis and function, autophagy is responsible for the lysosome-mediated degradation of damaged proteins and organelles; therefore, dysregulation of autophagy in humans can lead to a variety of diseases. The link between impaired autophagy and disease highlights the need to investigate possible interventions to address dysregulations. One possible intervention is hyperthermia, which is described in this protocol. To investigate these interventions, a method for absolute quantification of autophagosomal compartments is required that allows comparison of autophagosomal activity under different conditions. Existing methods such as western blotting and immunohistochemistry for analysing the location and relative abundance of intracellular proteins associated with autophagy, or transmission electron microscopy (TEM), which are either very time-consuming, expensive, or both, are less suitable for this purpose. The method described in this protocol allows the absolute quantification of autophagosomes per cell in human fibroblasts using the CYTO-ID® Autophagy Detection Kit after heat therapy compared to a control. The Cyto-ID® assay is based on the use of a specific dye that selectively stains autophagic compartments, combined with an additional Hoechst 33342 dye for nuclear staining. The subsequent recognition of these stained compartments by the Cytation Imager enables the software to determine the number of autophagosomes per nucleus in living cells. Additionally, this absolute quantification uses an image-based method, and the protocol is easy to use and not time-consuming. Furthermore, the method is not only suitable for heat therapy but can also be adapted to any other desired therapy or substance.
Key features
• Absolute quantification of autophagic compartments in living cells.
• Optimised protocol for the determination of autophagy in primary human skin fibroblasts.
• Allows the testing of active substances and treatments concerning autophagy.
• Imaging-based method for the determination of autophagy.
Keywords: Autophagy (自噬)Graphical overview
Background
As part of normal housekeeping, mammalian cells regularly replace damaged organelles or misfolded proteins to prevent potentially dangerous components from accumulating and overwhelming the cell. Autophagy removes and recycles this waste by isolating the targeted materials within a double-membrane vesicle called an autophagosome, which fuses with the lysosome. The fusion is facilitated by tethering factors that bind to proteins on the autophagosome and the lysosome [1]. After fusion with the lysosomes, the cellular waste is degraded by acidic lysosomal hydrolases (Figure 1). Disruption of the autophagy process is implicated in numerous human diseases and pathophysiological conditions, including neurodegenerative, infectious, autoimmune, cardiovascular, rheumatic, metabolic, pulmonary, and malignant diseases and ageing [2,3]. For this reason, it is important to find interventions such as treatment methods and/or substances that counteract impaired autophagy. Autophagy is known to be triggered under stress conditions such as starvation, hypoxia, DNA damage, ER stress, pathogen infection [4], and, in the case of this protocol, hyperthermia. The hyperthermia or heat treatment was carried out using an IRAcubator to induce autophagy. This device is a portable incubator that uses infrared-A radiation in the wavelength range from 780 to 1,400 nm, which heats the human fibroblasts to 39 °C. This is just one example of a possible treatment method for influencing autophagy. Regardless of the investigated intervention, however, a reliable and reproducible method is required for such measurements. Common methods for detecting autophagy are transmission electron microscopy (TEM) or western blot. However, TEM is very expensive and more suitable for analysing the location rather than the number of autophagosomes. Western blot requires a large amount of sample material, is very time-consuming, and expensive. Other methods such as the LC3 HiBiT reporter assay or other reporter systems are more suitable for cell lines than for primary cells. Another possible method, which was also used in our paper [5], could be the quantification by flow cytometry after staining with anti-LC3 FITC conjugated antibodies. However, this method is currently only established in our laboratory for peripheral blood mononuclear cells (PBMCs) and not for human fibroblasts.
The CYTO-ID® Autophagy Detection Kit used in this protocol contains a green dye that has been optimised by identifying titratable functional components that allow minimal staining of lysosomes while showing bright fluorescence when incorporated into pre-autophagosomes, autophagosomes, and autolysosomes. The kit also contains Hoechst dye for nuclear staining to enable the detection of autophagosomes per nucleus. In addition, the lysosomal inhibitor chloroquine can be added as a positive control. By inhibiting lysosomal degradation, the autophagosomal process is interrupted and autophagosomes accumulate (Figure 1). This means that in the wells that also contain chloroquine, a higher number of autophagosomes per nucleus should be seen if the staining has worked properly. For this reason, additional wells with added chloroquine were prepared for both conditions as a positive control for the assay.
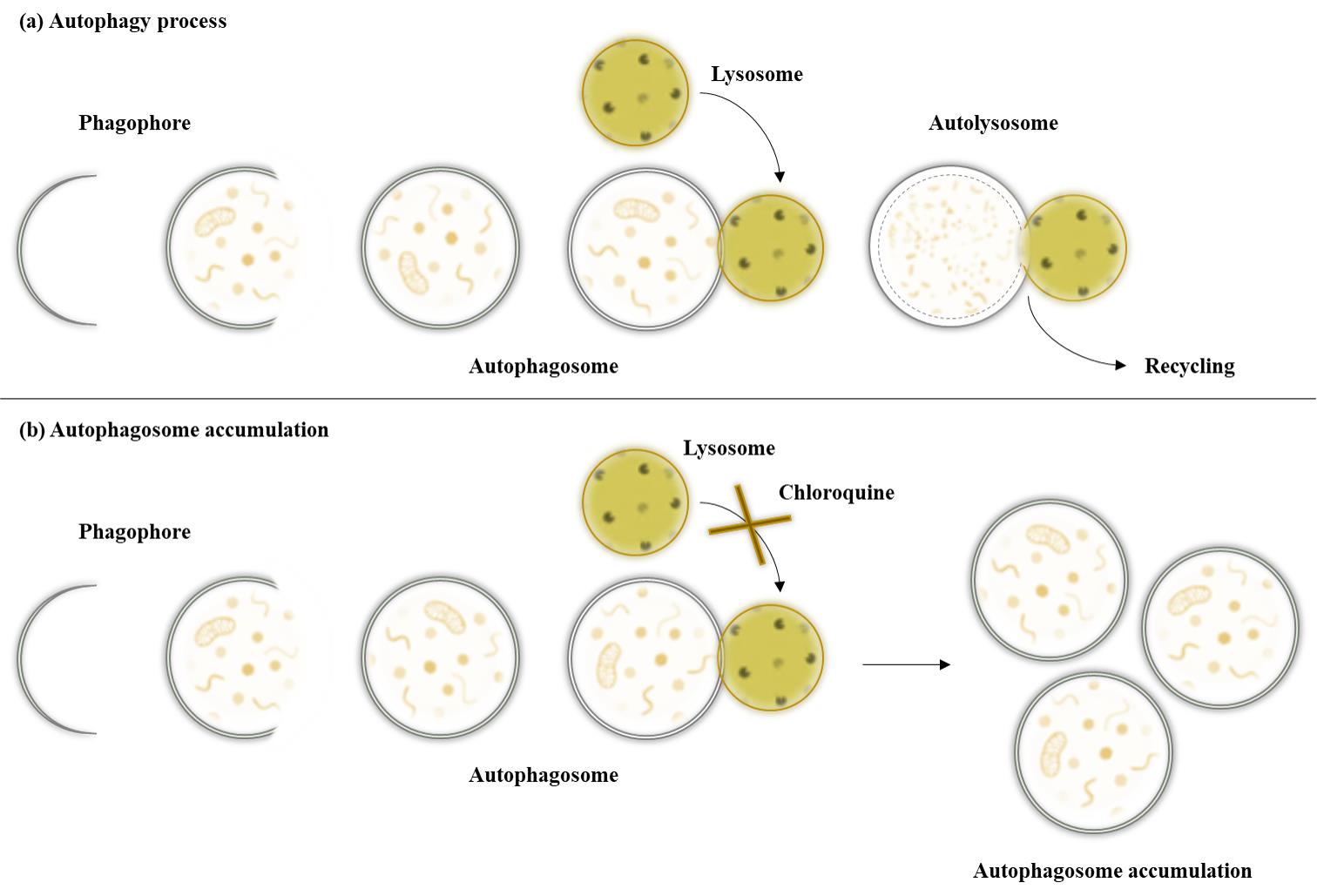
Figure 1. Autophagy pathway under different conditions. After maturation of the autophagosomes, the vesicles are either degraded by fusion with lysosomes or accumulate due to the inhibition of lysosomal fusion by chloroquine.
The advantages of this method are that it is easy to use and not time-consuming. Furthermore, it is not only suitable for heat therapy but can also be adapted to any other desired therapy or substance. It can also be adapted to other adherent cells such as HeLa cells. The method is not suitable for small, round cells such as peripheral blood mononuclear cells (PBMCs).
Materials and reagents
Biological materials
Primary human skin fibroblasts, prepuce of a healthy eight-year-old male donor, passage 08–09
Reagents
Fetal bovine serum (FBS) (Gibco, catalog number: 11573397)
Trypsin-EDTA (0.5%) (Gibco, catalog number: 10779413)
Chloroquine diphosphate (TOCRIS, catalog number 4109)
Dimethyl sulphoxide (DMSO) ≥ 99.5%, BioScience grade (Carl Roth, catalog number: A994.2)
Dulbecco’s modified Eagle’s medium, high glucose (DMEM) (Gibco, catalog number: 41966052)
Gentamycin (10 mg/mL) (Gibco, catalog number: 11500506)
Sodium chloride (NaCl) (Carl Roth, catalog number: 3957)
Di-sodium hydrogen phosphate dodecahydrate (Na2HPO4·12H2O) (Carl Roth, catalog number: N350)
Potassium dihydrogen phosphate (KH2PO4) (Carl Roth, catalog number: 3904)
Potassium chloride (KCl) (Carl Roth, catalog number: 6781)
CYTO-ID® Autophagy Detection Kit 2.0 (Enzo Life Sciences GmbH, catalog number: ENZ-KIT175-0200)
Solutions
1× Phosphate buffered saline (PBS) (see Recipes)
DMEM for fibroblasts (see Recipes)
Stop solution (see Recipes)
Cryomedium (see Recipes)
1× assay buffer (see Recipes)
Staining solution (see Recipes)
Recipes
1× Phosphate buffered saline (PBS)
Note: Adjust pH value to 7.4. Autoclave 10× PBS; for 1× PBS, dilute 10× stock 1:10 with deionized water (50 mL of 10× PBS + 450 mL of deionized water), autoclave, and store at room temperature.
Reagent Final concentration (10×) Quantity or volume (10×) NaCl 8.0% (w/v) 80.0 g KCl 0.2% (w/v) 2.0 g Na2HPO4·12H2O 1.78% (w/v) 17.8 g KH2PO4 0.24% (w/v) 2.4 g Deionized water n/a ad 1,000 mL Total (optional) n/a 1,000 mL DMEM for fibroblasts (medium)
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume DMEM 90.5% (v/v) 500 mL FBS 9.05% (v/v) 50 mL Gentamycin 0.45% (v/v) 2.5 mL Total (optional) 100% 552.5 mL Stop solution
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume 1× PBS 90.91% (v/v) 500 mL FBS 9.09% (v/v) 50 mL Total (optional) 100% 550 mL Cryomedium
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume FBS 90% (v/v) 45 mL DMSO 10% (v/v) 5 mL Total (optional) 100% 50 mL 1× assay buffer
Note: Store at 4 °C.
Reagent Final concentration Quantity or Volume 10× assay buffer (from CYTO-ID®) 1× 1 mL Deionized water 9 mL Total (optional) n/a 10 mL Staining solution (CYTO-ID®)
Note: Prepare immediately before use, do not store. All listed ingredients are part of the CYTO-ID® Autophagy Detection Kit 2.0.
Reagent Final concentration Quantity or Volume 1× assay buffer n/a 2.4 mL CYTO-ID® green detection reagent n/a 4.8 µL Hoechst 33342 Nuclear Stain n/a 2.4 µL Total (optional) n/a 2407.2 µL
Laboratory supplies
Centrifuge tubes, 15 and 50 mL (SARSTEDT, catalog numbers: 62.554.502, 62.547.254)
Reaction tube, 1.5 and 2 mL (SARSTEDT, catalog numbers: 72.706, 72.695.500)
CryoPure vial, 1.6 mL (SARSTEDT, catalog number: 72.380.992)
Pipette tips, 20, 200, and 1,000 μL (SARSTEDT, catalog numbers: 701.114.210, 70.3030, and 70.3050)
Serological pipettes, 5, 10, and 25 mL (SARSTEDT, catalog numbers: 86.1253.001, 86.1254.001, and 86.1685.001)
Cell culture plate, 96-well microplate (Greiner, catalog number: 655090)
Cell culture flask, T175, Cell+ (SARSTEDT, catalog number: 83.3912.302)
Equipment
Pipettes: Eppendorf Research® Plus 10 μL, 20 μL, 200 μL, 1,000 μL (Eppendorf, catalog numbers: 3123000020, 3123000039, 3123000055, 3123000063)
Multichannel microliter pipette Transferpette®, 20–200 µL (BRAND GMBH + CO.KG, catalog number: 9280173)
Pipetting aid: PipetBoy acu 2 (Integra Biosciences, catalog number: 155 000)
Neubauer counting chamber improved (Carl Roth, catalog number: PC72.1)
Inverted microscope: Primovert, Objektiv Plan-Achromat 4×/0.10, 10×/0.25 Ph1 (Carl Zeiss, catalog numbers: 415510-1100-000, 415500-1600-001, 415500-1605-001)
Water bath (GFL, catalog number: 1003)
Microcentrifuge Eppendorf 5424 (Eppendorf, catalog number: 05-400-005)
Swing out rotor centrifuge Eppendorf 5804 R (Eppendorf, catalog numbers: 5805000010, 5804709004)
Water purification system ELGA® PURELAB flex 3 (Veolia, catalog number: PF3XXXXM1)
pH meter FE20 FiveEasyTM (Mettler Toledo, catalog number: 30266626)
Heat treatment IRAcubator (Von Ardenne Institut für Angewandte Medizinische Forschung GmbH)
Incubator at 37 °C with 5% CO2, 90% humidity (HERA Cell 240, catalog number: 2510-413-01)
BioTek Cytation 1 cell imaging multimode reader, DAPI 377, 447; GFP 469, 525 (Agilent, model: CYT1AGV)
Software and datasets
Gen 5 Image Prime (3.09.07, 01/10/2020), license needed
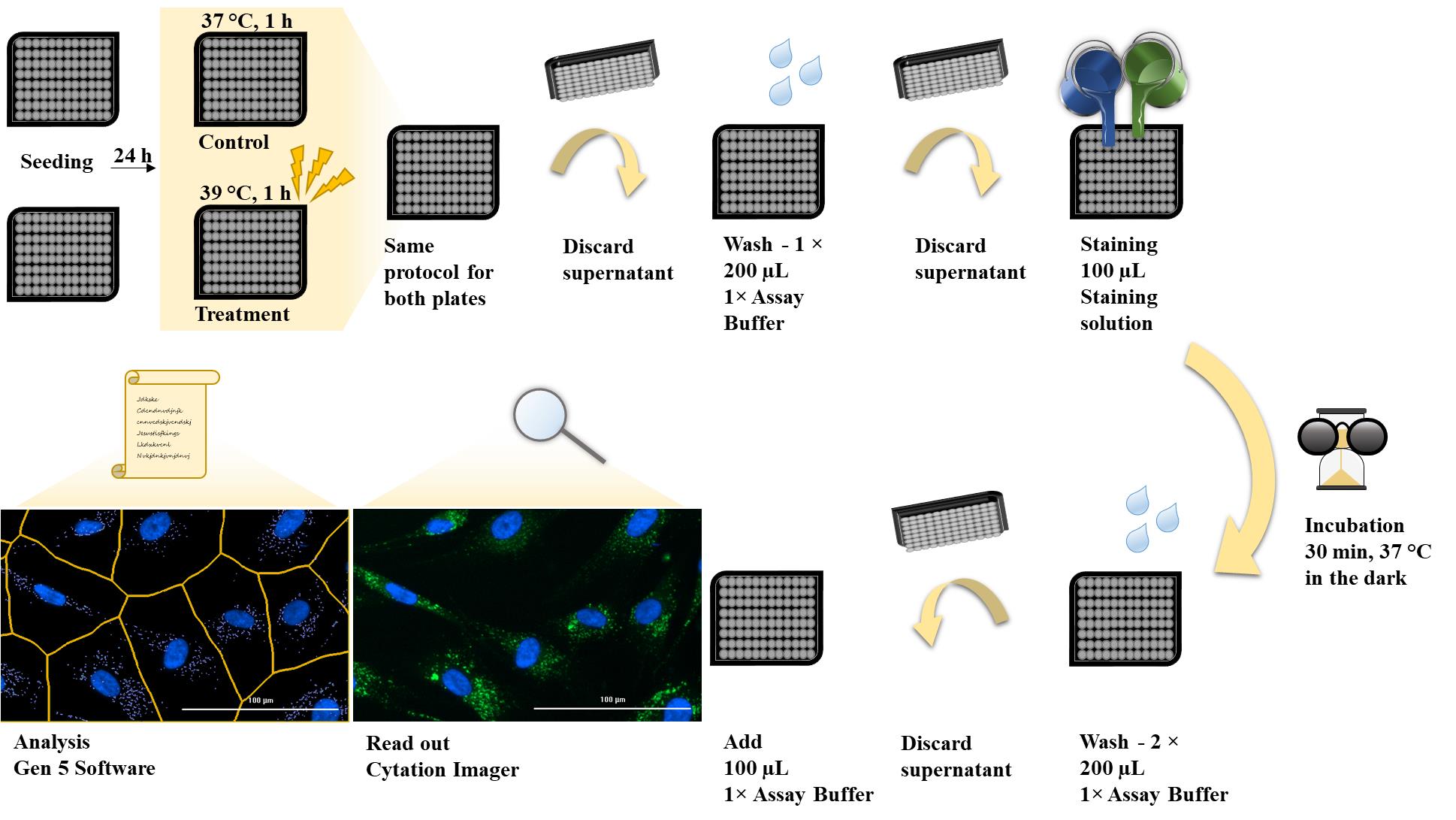
Procedure
文章信息
稿件历史记录
提交日期: Apr 2, 2024
接收日期: May 30, 2024
在线发布日期: Jun 23, 2024
出版日期: Jul 5, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Hochecker, B., Matt, K. C., Meßmer, A. L., Scherer, M. M. and Bergemann, J. (2024). Quantification of Autophagosomes in Human Fibroblasts Using Cyto-ID® Staining and Cytation Imaging. Bio-protocol 14(13): e5025. DOI: 10.21769/BioProtoc.5025.
分类
细胞生物学 > 细胞成像 > 活细胞成像
细胞生物学 > 细胞活力 > 细胞死亡
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link