- EN - English
- CN - 中文
Reversible Photoregulation of Cell–Cell Adhesions With Opto-E-cadherin
利用Opto-E-cadherin可逆光调控细胞间粘附
发布: 2024年05月20日第14卷第10期 DOI: 10.21769/BioProtoc.4995 浏览次数: 2770
评审: Munenori IshibashiJamie A. Davies Anonymous reviewer(s)
Abstract
The cell–cell adhesion molecule E-cadherin has been intensively studied due to its prevalence in tissue function and its spatiotemporal regulation during epithelial-to-mesenchymal cell transition. Nonetheless, regulating and studying the dynamics of it has proven challenging. We developed a photoswitchable version of E-cadherin, named opto-E-cadherin, which can be toggled OFF with blue light illumination and back ON in the dark. Herein, we describe easy-to-use methods to test and characterise opto-E-cadherin cell clones for downstream experiments.
Key features
• This protocol describes how to implement optogenetic cell–cell adhesion molecules effectively (described here on the basis of opto-E-cadherin), while highlighting possible pitfalls.
• Utilises equipment commonly found in most laboratories with high ease of use.
• Phenotype screening is easy and done within a few hours (comparison of cell clusters in the dark vs. blue light in an aggregation assay).
• Three different functionality assay systems are described.
• After the cell line is established, all experiments can be performed within three days.
Keywords: Photoswitchable (光开关)Graphical overview
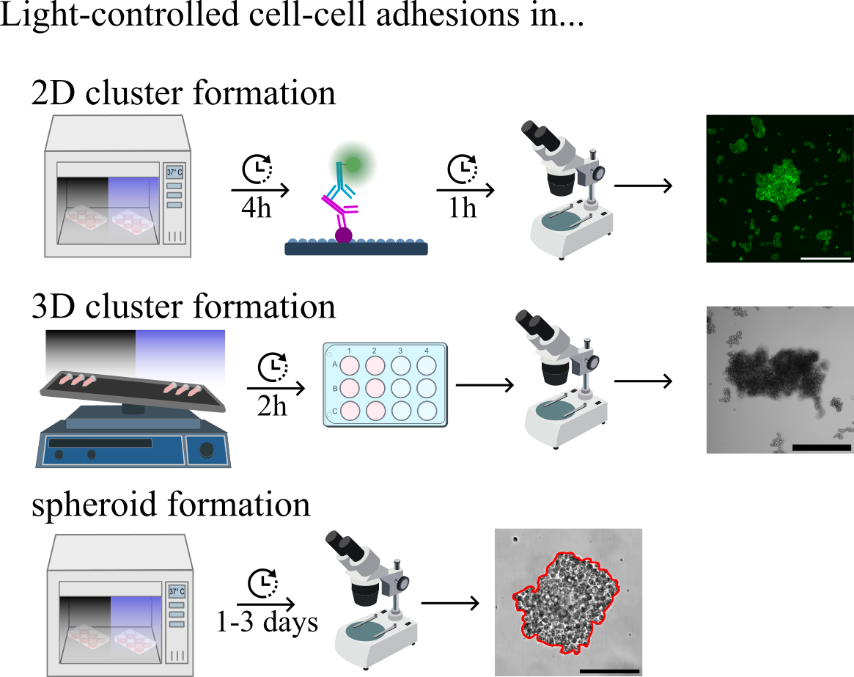
Analysis and characterisation of light-dependent opto-E-cadherin expressing cells
Background
In multicellular organisms, many essential biological processes such as embryonic development, tissue integrity, collective cell behaviour, and migration, are regulated by cell–cell adhesions [1], while aberrations play a key factor in diseases such as arthritis, neuronal dystrophy, and tumour progression [2–4]. Epithelial cadherin (E-cadherin) is a well-known cell-adhesion protein. Upon binding of two E-cadherin molecules on neighbouring cells, intracellular proteins are recruited to the cytoplasmic tail and connect directly to the cytoskeleton, affecting transcriptional regulation [5]. Its loss of expression or change in function may lead to epithelial-to-mesenchymal transition (EMT), tumour development, and metastasis [6,7]. In healthy tissues, EMT of cells is dynamically and locally regulated, which may be reversed by mesenchymal-to-epithelial transition (MET). At the same time, EMT plays a central role in gastrulation and wound healing in adults [8]. Attempts to study the underlying mechanisms have relied on gain- or loss-of-function experiments, inhibition with antibodies, or the removal of Ca2+ ions, necessary for the homophilic interaction between E-cadherins [9–11]. However, none of these methods fully captures the spatio-temporal regulation in cell–cell adhesion during EMT. Attempts to mimic cell–cell adhesions through chemical modifications of the cell surface with synthetic cell adhesion molecules were neither sustainable nor dynamic [12,13]. To gain spatiotemporal control over cell–cell adhesions, various chemo-optogenetic [14,15] and optogenetic tools that utilise light to dissociate cell adhesions [16] and create artificial cell–cell adhesions have been successfully developed [17–21].
More recently, we developed a blue light switchable E-cadherin molecule (opto-E-cad), which allows us to reversibly and dynamically switch between ON and OFF states of E-cadherin [22]. In opto-E-cad, a light-oxygen-voltage sensing domain (LOV2) is embedded in the extracellular domain of E-cadherin such that, in the dark, cell–cell adhesion can form. Upon blue light absorption by its cofactor FMN or FAD, the LOV2 domain undergoes a conformational change, disturbing a critical Ca2+ binding site and turning OFF the opto-E-cad-based adhesions. In the dark, these conformational changes reverse, and the cell–cell adhesions can form again. Overall, opto-E-cad is fully genetically codable and provides bidirectional control over cell–cell adhesions with high spatiotemporal precision. At the same time, opto-E-cad allows us to link optogenetics to cellular signalling associated with E-cadherin-based cell–cell adhesions, providing a unique tool to suitably study EMT and MET processes and their impact on development and disease progression. As proof of concept, we have demonstrated the feasibility and ease-of-use of opto-E-cad in several invasion assays either with single cells and spheroids in vitro and in vivo, as well as its influence on transcriptional regulation similar to observed changes in gene expression during EMT via q-RT PCR [23]. When considering E-cadherin, varying expression levels and adhesion strengths are a determining factor in its function [24,25]. Thus, straightforward methods to characterise cells with different levels of opto-E-cad expression are necessary, which we describe here.
Materials and reagents
Biological materials
MDA-MB-231 human breast adenocarcinoma epithelial cells (American Type Culture Cells, catalog number: HTB-26)
L929 mouse fibroblast cells derivative of strain L (American Type Culture Cells, catalog number: CCL-1)
HeLa human cervix adenocarcinoma epithelial cells (American Type Culture Cells, catalog number: CCL-2)
A431D (Magaret J. Wheelock, origin: University of Toledo, Ohio [26])
Opto-E-cad-GFP plasmid (Addgene, catalog number: 203327)
Reagents
Dulbecco’s modified Eagle medium (DMEM) (PAN Biotech, catalog number: 04-03591)
Fetal bovine serum (FBS) (PAN Biotech, catalog number: P30-3031)
Penicillin-streptomycin (P/S) (Gibco, catalog number: 15140122)
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (Sigma-Aldrich, catalog number: H4304)
Lipofectamine 3000 (Thermo Fisher, catalog number: L300001)
Geneticin (G418) (Roche, catalog number: 4727878001)
Phosphate-buffered saline (PBS) (Gibco, catalog number 18912014)
StemPro Accutase (Thermo Fisher, catalog number: A1110501)
Flavin adenine dinucleotide (FAD) (Carl Roth, catalog number: 6833)
Paraformaldehyde (PFA) (Sigma-Aldrich, catalog number: 158127)
Hank’s buffered salt solution (HBSS) (Gibco, catalog number: 12549069)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7030)
Calcium chloride (CaCl2) (Carl Roth, catalog number: A119.1)
Ethylenediaminetetraacetic acid (EDTA) (Carl Roth, catalog number: 80423)
Triton X-100 (PanReac AppliChem, catalog number: not available)
Hoechst 3342 (Invitrogen, catalog number: H3570)
Phalloidin iFluor-594 (Abcam, catalog number: ab176757)
Cell tracker Green BODIPY dye (Invitrogen, catalog number: C2102)
Fluoromount GTM (Invitrogen, catalog number: 00595802)
Methyl cellulose (viscosity 15cP) (Sigma-Aldrich, catalog number: M7027)
Recipes
Growth medium
Reagent Final concentration Quantity or Volume DMEM n/a 435 mL FBS 10% 50 mL P/S (10,000 U/mL) 1% 5 mL HEPES (625 mM) 12.5 mM 10 mL Total n/a 500 mL Spheroid medium
Reagent Final concentration Quantity or Volume DMEM n/a 470 mL FBS 3% 15 mL P/S (10,000 U/mL) 1% 5 mL HEPES (625 mM) 12.5 mM 10 mL Methyl cellulose (viscosity 15cP) 0.66% 3.3 g Total n/a 500 mL Note: Filter FBS through a 0.22 μm filter before adding it to DMEM for growth and spheroid medium.
Laboratory supplies
T-25 flasks (Sarstedt, catalog number: 83.3910.002)
6-well microplates (Greiner Bio-One, catalog number: 657160)
12-well microplates (Greiner Bio-One, catalog number: 665180)
96-well F-bottom microplates (Greiner Bio-One, catalog number: 655087)
96-well U-bottom suspension microplates (Greiner Bio-One, catalog number: 650185)
DNA LoBind microfuge tubes (Eppendorf, catalog number: 022431021)
24 mm × 24 mm glass coverslips (Roth, catalog number: HKE8.1)
Microscope slides (Thermo Scientific, catalog number: 15457544)
10 μL TipOne® pipette tips (Starlab, catalog number: S1111-3700-C)
200 μL TipOne® pipette tips (Starlab, catalog number: S1113-1706-C)
1,000 μL TipOne® pipette tips (Starlab, catalog number: S1111-6701-C)
Equipment
10 μL NeoLab pipette (Eppendorf, catalog number: A-0861)
100 μL NeoLab pipette (Eppendorf, catalog number: A-0864)
1,000 μL NeoLab pipette (Eppendorf, catalog number: A-0867)
CO2 incubator (PHCbi, model: MCO-170AICUVD-PE)
CO2 incubator with custom drilled cable inlets for LED panels (Thermo Fisher, model: Midi CO2 40L 3403)
Herasafe cabinet (Thermo Fisher, model: Herasafe 2030i)
Ultra centrifuge (Eppendorf, model: 5804)
Automated cell counter (Bio-Rad, model: TC20)
Cell sorter (BD Biosciences, model: FACSAria Fusion)
CLF flora LED (CLF Plant Climatics GmbH, model: not available)
Custom made LED panels and control modules. Equivalent self-made products can be found here [27]
Power meter (Thorlabs, catalog number: PM16-120)
3D orbital shaker (Grant-Bio, model: PSM3D)
Dmi8 brightfield microscope (Leica, model: Dmi8 automated)
Software and datasets
LAS X v.3.7.1.21655 (Leica, 25/01/2024)
ImageJ v.1.53f51 (ImageJ, 02/03/2023)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Raab, C. A. and Wegner, S. V. (2024). Reversible Photoregulation of Cell–Cell Adhesions With Opto-E-cadherin. Bio-protocol 14(10): e4995. DOI: 10.21769/BioProtoc.4995.
分类
细胞生物学 > 基于细胞的分析方法 > 细胞粘附
分子生物学 > 蛋白质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link












