- EN - English
- CN - 中文
A Standardized Protocol for Extraction and Homogenization of Ocular Tissues
眼组织提取与均质化的标准化方案
发布: 2024年05月20日第14卷第10期 DOI: 10.21769/BioProtoc.4988 浏览次数: 2975
评审: Vivien J. Coulson-ThomasAnonymous reviewer(s)
Abstract
The eye is a complex organ composed of multiple tissues in anterior and posterior eye segments. Malfunctions of any of these tissues can lead to ocular diseases and loss of vision. A detailed understanding of the ocular anatomy and physiology in animal models and humans contributes to the development of ocular drugs by enabling studies on drug delivery and clearance routes, pharmacokinetics, and toxicity. This protocol provides step-by-step instructions for the extraction and homogenization of ocular tissues for enzymatic and proteomics analyses.
Key features
• Suitable protocol for the extraction and isolation of ocular tissue from humans and laboratory animals (rabbit, pig, rat, mouse) while minimizing cross-contamination.
• Hard or soft tissue homogenates can be prepared efficiently using a Bead Ruptor homogenizer.
• Allows to determine the protein contents in prepared homogenates.
Keywords: Eye (眼)Graphical overview
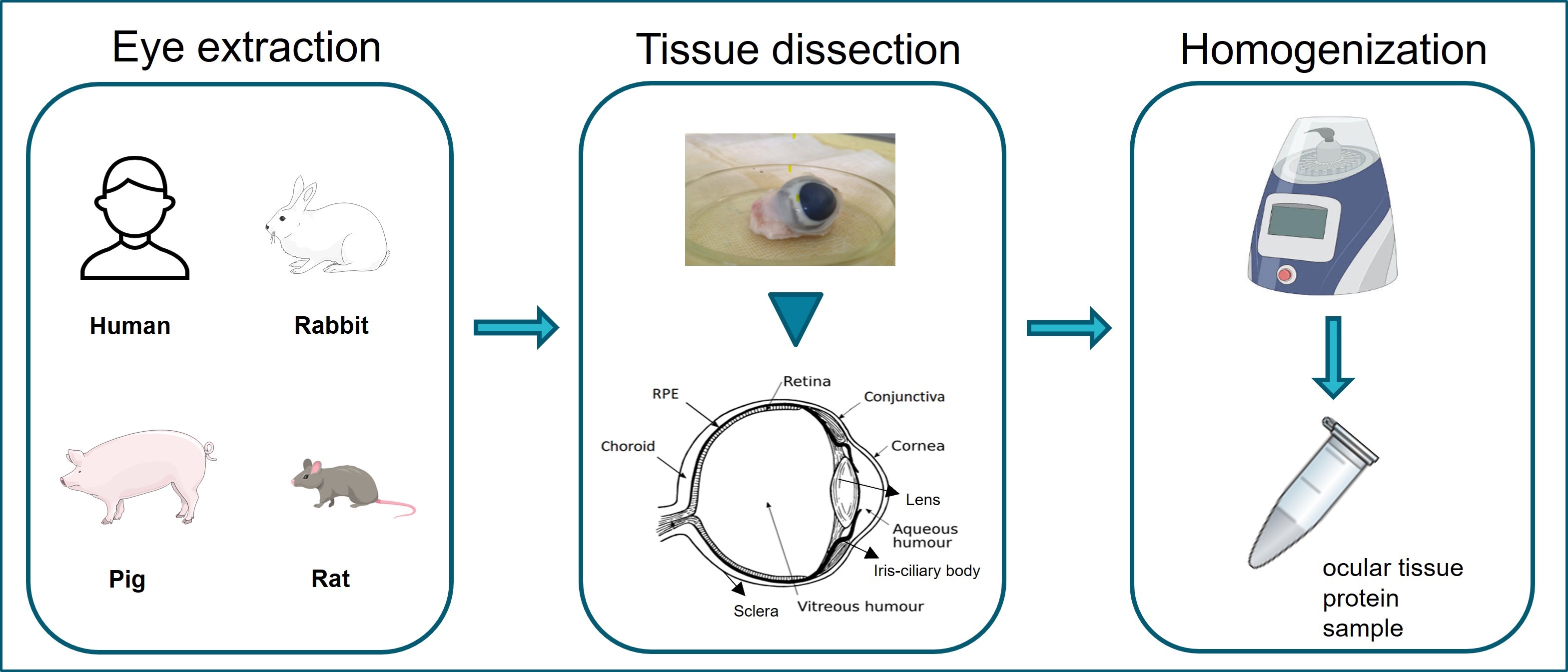
Graphical overview of ocular tissue extraction starting from eye enucleation from human or laboratory animal samples. Extraction and separation of ocular tissues are shown in tissue dissection. Homogenization of ocular tissues collected using the Bead Ruptor Elite homogenizer.
Background
Ocular diseases lead to vision loss in the aging global population [1,2], decreasing quality of life and increasing costs of health care [3]. Ocular drug development seeks to alleviate eye diseases by developing improved ocular therapeutics [4]. With that intention, a detailed understanding of ocular compartments, tissues in the anterior and posterior segments, and barriers is required [5,6].
The eye encompasses multiple tissues that individually play a significant role in the vision [7]. Anatomically, the eyeball is composed of a smaller anterior and a larger posterior segment [8]. The anterior tissues include the conjunctiva, cornea, aqueous humor, lens, iris, and ciliary body. The posterior segment contains vitreous humor, retinal pigment epithelium (RPE), neural retina, choroid, and sclera [9].
Isolation of contamination-free (devoid of neighboring/cross tissues and foreign-substance contaminants that could interfere with subsequent analysis or experiments) individual ocular tissues is challenging [3] due to the small tissue sizes and complexity. This protocol aims to provide details for (i) the separation and isolation of the ocular tissues, (ii) the preparation of ocular tissue homogenates, and (iii) the quantification of protein contents in prepared homogenates, e.g., enzymatic and proteomic analyses. Minimizing cross-contamination from anatomically adjacent tissues and preserving protein integrity is of fundamental importance.
This optimized protocol allows the preparation of tissue homogenates from fresh or frozen eyeballs. The procedure begins with the aspiration of the aqueous humor. Thereafter, all anterior eye tissues (conjunctiva, cornea, lens, iris, and ciliary body) can be individually separated from each other, leaving the vitreous exposed for collection. Further steps allow the collection of posterior eye tissues (retina, RPE, choroid, and sclera). The differences in tissue hardness influence the homogenization steps, which are described in detail.
Materials and reagents
Biological material
Human or laboratory animal (e.g., rabbit or pig) eyeballs. This protocol is equally suitable for rat and mouse eyes, but dissection and tissue isolation are recommended to be performed under the microscope
Reagents
Dulbecco's phosphate-buffered saline, 10× (DPBS) (Thermo Fisher, catalog number: 14200166)
Potassium phosphate buffer (PBS) (Sigma-Aldrich, catalog number: P3813)
Sterile water (Baxter, catalog number: KKF7113)
Ethanol (Sigma-Aldrich, catalog number: 64-17-5)
Syringe 1 mL (Luer Slip IV Syringes, catalog number: FWC345)
26 G needle 1/2″ (0.45 × 12 mm) (Medoject, catalog number: CH26012)
Scissors: Sharp straight (115 mm 122-010) (Elcon Scissor Tenotomy Stevens Straight, catalog number: 547456), curved pointed scissors (115 mm, curved, pointed St) (Elcon Iris Scissors, catalog number: ELC122011)
Beakers
Scalpel (Swann-Morton sterile disposable) (Sigma-Aldrich, catalog number: S2771-100EA)
Small brushes to scrape RPE; size 4–6 mm (round or flat depending on the size of eye and species; for rats and mice, smaller sizes are more suitable)
Eppendorf tubes, 1.5/2 mL sterile [Reaction tube, 1.5 mL (SARSTEDT, catalog number: 72.690.001); reaction tube, 2 mL (SARSTEDT, catalog number: 72.691)]
50 mL Falcon tubes (Screw cap, 114 × 28 mm) (SARSTEDT, catalog number: 62.547.254)
Pipettes and pipette tips [FinnpipetteTM F2 Good Laboratory Pipetting (GLP) Kit] (Thermo Fisher Scientific, catalog number: 4700880)
Bradford assay kit [10] (Bio-Rad Laboratories, catalog number: 5000205)
2 mL reinforced Polypropylene, 2.8 mm ceramic beads tube (Omni Internationals Kennesaw, catalog number: 19-628D)
Costar® 6-well flat-bottom plate, clear, polystyrene (Costar, catalog number: 38015)
Petri dishes, 92 × 16 mm, transparent (SARSTEDT, catalog number: 82.1472)
Filter papers (Whatman® qualitative filter paper) (Sigma-Aldrich, catalog number: WHA1001929)
Note: Use only sterile and clean equipment during the dissection. Also, tissue isolation should be performed in a sterile environment by cleaning surfaces and equipment thoroughly, followed by disinfection and sterilization.
Equipment
Olympus CK2 inverted phase contrast microscope (Microscope Central, model: ULWCD 0.3 N.A.)
Microcentrifuge (Thermo Fisher Scientific, model: Heraeus Biofuge 24 Place)
Bead Ruptor Elite homogenizer Omni Internationals (Kennesaw, GA, USA)
2 mL reinforced tubes filled with 2.8 mm diameter ceramic beads (Omni Internationals. Kennesaw, GA, USA)
Microplate reader (PerkinElmer Wallac, model: Victor2)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Hammid, A. (2024). A Standardized Protocol for Extraction and Homogenization of Ocular Tissues. Bio-protocol 14(10): e4988. DOI: 10.21769/BioProtoc.4988.
分类
细胞生物学 > 细胞分离和培养 > 细胞分离
生物化学 > 蛋白质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link