- EN - English
- CN - 中文
Preparing and Evaluating the Stability of Therapeutically Relevant Oligonucleotide Duplexes
制备并评估治疗相关寡核苷酸双链体的稳定性
(§ Technical contact) 发布: 2024年04月20日第14卷第8期 DOI: 10.21769/BioProtoc.4975 浏览次数: 4309
评审: Chiara AmbrogioWendy Leanne HempstockCatherine HurdAnonymous reviewer(s)

相关实验方案
活细胞中单拷贝基因内源性 RNA 聚合酶 II 磷酸化的可视化、量化和建模
Linda S. Forero-Quintero [...] Timothy J. Stasevich
2022年08月05日 2952 阅读
Abstract
The field of oligonucleotide therapeutics is rapidly advancing, particularly for combating orphan diseases and cancer. However, the intrinsic instability of oligonucleotides, especially RNA, poses a substantial challenge in the face of the harsh conditions encountered intracellularly and in circulation. Therefore, evaluating the stability of oligos in serum is of great significance when developing oligonucleotide therapeutics. This protocol outlines a dependable and reproducible method for preparing oligonucleotide duplexes, coupled with confirmation by gel electrophoresis. Subsequently, the protocol defines a mechanism to assess the stability of the oligo duplexes in serum. This protocol seeks to establish a standardized reference for researchers, enabling them to compare the impact of various modifications on oligo stability and assess the degradation kinetics effectively.
Key features
• Adaptable for use with small interfering RNA (siRNA), microRNA (miRNA), antisense oligonucleotides (ASOs), and other unmodified and modified oligonucleotides.
• Does not necessitate any Biological Safety Level clearance and offers a rapid, cost-effective, and entirely in vitro procedure.
• Allows researchers to evaluate multiple modification patterns that, when coupled with targeting activity, allow for selecting the best modification pattern prior to in vivo analysis.
Keywords: Serum (血清)Graphical overview
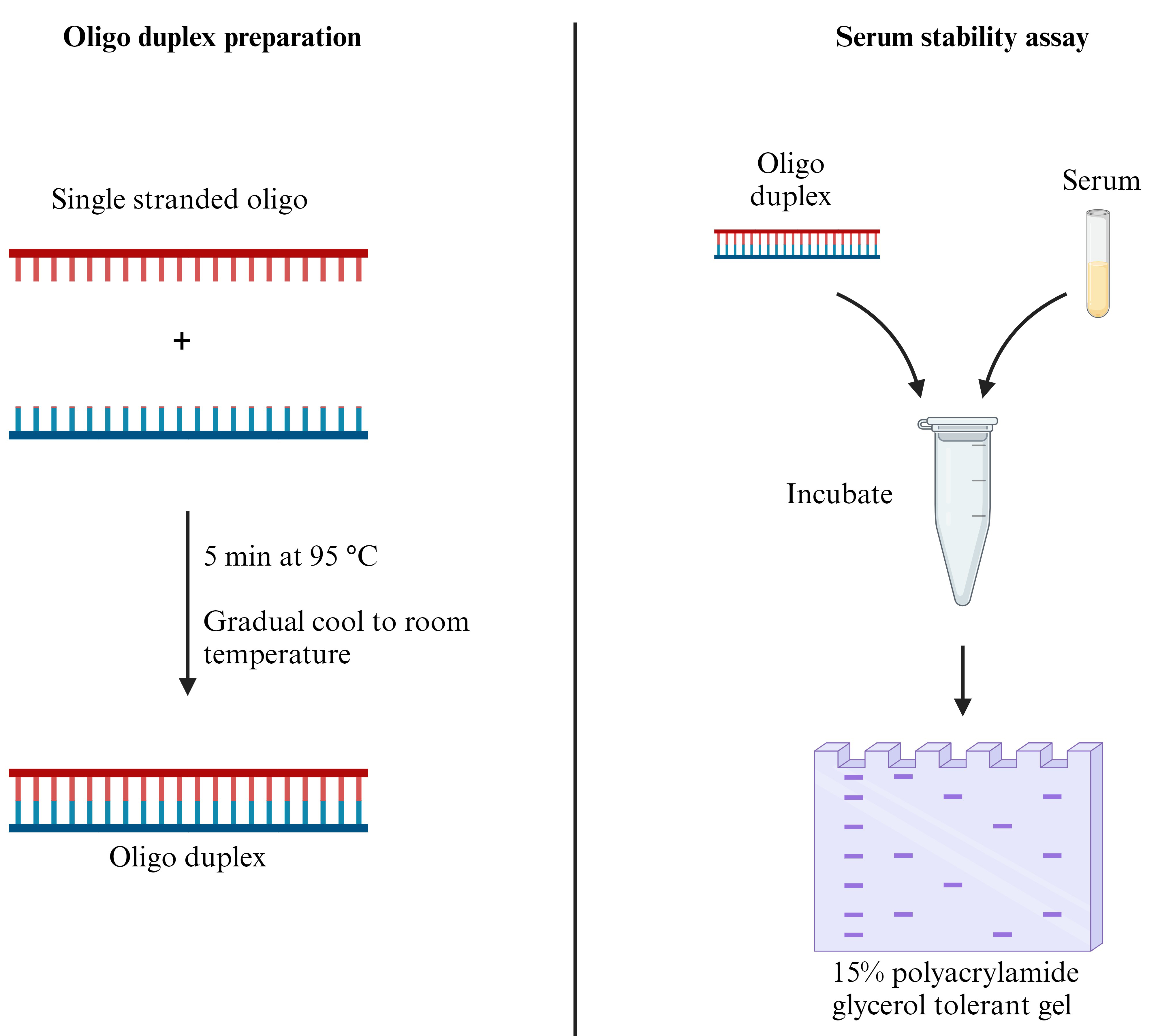
Background
The field of RNA therapeutics holds promise for applications in various diseases. However, the inherent instability of RNA poses a major obstacle, especially due to the harsh conditions encountered during circulation [1]. Therefore, a protocol for rapid in vitro evaluation of RNA stability in serum holds critical significance. This protocol presents a reliable and reproducible method for preparing oligo duplexes followed by the assessment of their stability in serum.
Fetal bovine serum (FBS) is used extensively in research settings as an additive to basal growth medium for cell and tissue culture applications. It serves as a rich source of proteins and growth factors that are vital for supporting cell growth in a cultured environment. FBS contains over 1,000 different components, including growth and attachment factors, lipids, hormones, essential nutrients, energy sources, electrolytes, carriers, and enzymes [2]. Notably, FBS also contains nucleases, which cause degradation of oligonucleotides over time [3,4]. Thus, FBS replicates a surrogate for the conditions faced by oligonucleotides during their circulation in the bloodstream, potentially even more rigorous than those encountered in circulation.
RNA instability, coupled with potential immunogenic effects associated with unmodified RNAs, are critical challenges in the development of RNA-based therapies. Unmodified oligos face rapid degradation by nucleases and elicit immunogenic effects, limiting their efficacy and necessitating high and frequent dosing in vivo [1]. Various chemical modifications, such as 2'-O-methyl and 2'-fluoro modifications to the ribose and phosphorothioate substitutions to the backbone, have been employed to enhance RNA stability [5–7]. Ribose modifications improve binding affinity and nuclease protection, while phosphorothioate bonds confer resistance to exonucleases [8]. The 2'-O-methyl modification also mitigates immune system stimulation triggered by delivered RNA. Although these modifications are advantageous, each RNA requires a unique pattern of modifications along both sense and antisense strands to limit degradation while maintaining target engagement. The protocol provided here allows one to evaluate "the stability" of multiple modification patterns and, when coupled with gene expression analysis (qRT-PCR or western), the researcher can easily identify the optimal modification pattern for a particular RNA.
Due to blood-born nucleases, these RNA modifications are essential, especially when the RNA is delivered using direct ligand conjugation [9–11]. Nonetheless, this protocol's relevance extends to studies involving therapeutic RNA encapsulation and studies using transfection of modified miRNA and siRNA, which are subject to intracellular degradation, effects that can be evaluated using this simple in vitro protocol.
While the concept of serum stability is not novel, the methodologies employed by researchers tend to exhibit variability [12,13]. This protocol aims to serve as a standardized reference for researchers. By utilizing this protocol, researchers can effectively compare the impact of various modifications on the stability of various oligos and evaluate the degradation kinetics of oligonucleotide therapeutics.
Materials and reagents
Biological materials
Fetal bovine serum Premium (Bio-Techne, catalog number: S11150)
Oligonucleotides (Integrated DNA technologies or other relevant source)
Complementary sense and antisense strands
Options to add modified bases including 2'-O-methyl and 2'-fluoro nucleotides, modifications to the backbone, including phosphorothioate backbone modification, replacing phosphodiester bonds in the oligo backbone at specific locations, or other modifications.
Reagents
Nuclease-free water (Invitrogen, catalog number: AM9932)
Tris base, molecular biology grade (Millipore Sigma, catalog number: 648310)
Concentrated hydrochloric acid (HCl) (Fisher Chemical, catalog number: SA49)
Sodium chloride (NaCl) (Millipore Sigma, catalog number: S9888)
EDTA (0.5 M), pH 8.0, RNase-free (Invitrogen, catalog number: AM9260G)
Taurine (Millipore Sigma, catalog number: T0625)
Acrylamide/Bis solution 29:1 (30%) (Bio-Rad, catalog number: 1610156)
Ammonium persulfate (APS) (Millipore Sigma, catalog number: A3678)
TEMED (tetramethylethylenediamine) (Bio-Rad, catalog number: 1610800)
2× RNA loading dye (Thermo Scientific, catalog number: R0641)
GelRed nucleic acid gel stain (Fisher Scientific, Biotium, catalog number: NC9594719)
Phosphate buffered saline (PBS) (Fisher Scientific, catalog number: SH30256FS)
Solutions
NaCl (2.5 M) (see Recipes)
1 M Tris, pH 7.5-8.0 (see Recipes)
10× annealing buffer (see Recipes)
20× glycerol-tolerant gel buffer (see Recipes)
15% polyacrylamide glycerol-tolerant gel (see Recipes)
Recipes
NaCl (2.5 M)
Reagent Final concentration Quantity NaCl (powder) 2.5 M 1.46 g Nuclease-free H2O n/a 10 mL Total 2.5 M 10 mL 1 M Tris, pH 7.5-8.0
Reagent Final concentration Quantity Tris base 1M 121.1 g Nuclease-free H2O n/a 800 mL Concentrated HCl n/a 65 mL Nuclease-free H2O n/a Add to bring final volume to 1 L Total n/a 1 L 10× annealing buffer
Reagent Final concentration Quantity Tris, pH 7.5–8.0 (1 M) (Recipe 2) 100 mM 400 µL NaCl (2.5 M) (Recipe 1) 500 mM 800 µL EDTA (0.5 M) 10 mM 80 µL Nuclease-free H2O n/a 2,720 µL Total n/a 4 mL Store at -20 °C
20× glycerol-tolerant gel buffer
Reagent Final concentration Quantity Tris base 1.78 M 216 g Taurine 570 mM 72 g EDTA (0.5 M) 1 mM 2 mL Nuclease-free H2O n/a Bring to 1,000 mL Total n/a 1,000 mL Store at 4 °C
15% polyacrylamide glycerol-tolerant gel (see Note 4)
Reagent Final concentration Quantity Acrylamide/Bis solution 29:1 (30%) 15% 7.5 mL Glycerol-tolerant gel buffer (20×) (Recipe 4) 1× 750 µL Ammonium persulfate (10%) 0.1% 150 µL TEMED 1% 15 µL Nuclease-free H2O n/a 6.6 mL Total n/a 900 mL
Laboratory supplies
Standard pipette tips with a volume capacity of 2 µL, 20 µL, 200 µL, and 1 mL
Manual pipettes set of 2, 20, 200, and 1,000 µL (Mettler-Toledo, Rainin, catalog number: 17014393, 17014392, 17014391, and 17014382 or similar)
Premium microcentrifuge tubes 1.5 mL (Fisherbrand, catalog number: 05-408-129, or similar RNase-free microcentrifuge tubes)
Bel-Art® gel staining box with cover (Millipore Sigma, catalog number: BAF135511000-1EA)
Equipment
Standard dry block heaters (VWR, catalog number: 75838-318)
Criterion empty cassettes (Bio-Rad, catalog number: 3459901)
Criterion cell and PowerPac basic power supply (Bio-Rad, catalog number: 1656019)
VWR® compact UV transilluminator (VWR, catalog number: 76407-432)
Laboratory shaker (Reliable Scientific, model: 55)
High-resolution digital camera (iPhone, Android, or other cell phone cameras are acceptable)
Software and datasets
ImageJ is a Java-based (runs on Mac OS X, Linux, and Windows) freeware available for download at: http://rsb.info.nih.gov/ij/
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Iyer, S. G. and Kasinski, A. L. (2024). Preparing and Evaluating the Stability of Therapeutically Relevant Oligonucleotide Duplexes. Bio-protocol 14(8): e4975. DOI: 10.21769/BioProtoc.4975.
分类
生物化学 > RNA > 单分子活性
分子生物学 > RNA > miRNA 干扰
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link