- EN - English
- CN - 中文
Protocol for the Implantation of Scaffolds in a Humanized Mouse Cutaneous Excisional Wound Healing Model
人源化小鼠切割性伤口愈合模型中植入支架的方法
发布: 2024年09月20日第14卷第18期 DOI: 10.21769/BioProtoc.4974 浏览次数: 1469
评审: Vivien J. Coulson-ThomasSudhir VermaTarsis Gesteira Ferreira
Abstract
Tissue-engineered constructs combine the mechanical properties of biomaterials with biological agents to serve as scaffolds that direct the wound-healing process and promote tissue regeneration. A limitation to studying wound healing in vivo is that mouse skin contracts to heal rather than exhibiting granulation tissue formation and epithelialization like human skin. Therefore, it became necessary to develop a mouse model to better recapitulate human wound healing. The first splinted excisional wound healing model in mice, described in 2004, utilized silicone splints to prevent skin contracture. This model has been used to test a variety of wound healing strategies; however, to our knowledge, this model has not been adapted to test the effect of implants on wound healing. In our established protocol, circular bilateral excisional wounds are made on the mouse’s dorsum. A circular implant made of porous polyethylene is sutured to the skin within the wound. A thin, donut-shaped silicone splint is secured to the skin surrounding the wound, and a thick, donut-shaped splint is placed on top to tent the wound dressing. Finally, the mouse’s abdomen is wrapped in a bandage and tape to protect the implants. Our protocol offers a significant enhancement to the existing model by enabling the testing of implants for wound healing, as well as using an additional splint that prevents direct contact between the wound dressing and the wound bed. This model can be used to study tissue-engineered implant designs in a relatively low-cost, simple, and high-throughput manner before advancing to larger animal studies.
Key features
• Builds upon methods developed by Galiano et al. [1] and extends their application to include scaffold testing.
• Utilizes a construct that protects wounds, thereby enabling unaffected wound healing.
• Can be adapted to test a wide variety of biomaterials for wound healing.
• Describes dressing details and exact methodologies that prevent animals from interfering with wound healing.
Keywords: Wound healing (伤口愈合)Background
Non-healing wounds, chronic ulcers, and significant anatomical defects pose important challenges to both healthcare providers and scientists. Current wound healing research encompasses a range of innovative strategies, including tissue-engineered constructs, laser therapy, cell therapy, and platelet therapy [2]. These investigations are driven by the recognition that interactions between various cell types within the wound microenvironment demand a shift from in vitro to more complex animal models.
Although rabbits and pigs have historically served as valuable subjects in wound healing research, rodents have emerged as the most commonly utilized animal models due to their relatively low cost and small size [3]. Rodent and human skin differ in several ways, but the most significant is the presence of the rodent subcutaneous panniculus carnosus, which promotes healing through contraction [4]. In contrast, human skin relies on granulation and proliferation for healing [4]. Therefore, extensive efforts have been devoted to replicating human physiology in rodent models.
The current murine excisional wound model, first introduced in 2004, addressed the issue of contraction by incorporating a splint [1]. In this model, circular excisional wounds are created on the mouse dorsum, and donut-shaped silicone splints are centered around the wounds and secured to the skin. The addition of the splint prolongs wound healing by preventing contraction while yielding neo-epithelization that more closely resembles native tissue. Researchers have employed this model in a variety of settings including ischemic wounds, chronic ulcers, and hypertrophic scarring [5–10]. In 2023, Fischer et al. highlighted data analysis and splint troubleshooting; however, this publication still does not describe how to perform implant placement in the wound [11].
Although the existing model has been used successfully, there is a noticeable absence of publications demonstrating its applicability in testing a tissue-engineered, 3D implant that promotes wound healing. In this paper, we present two modifications to the splinted excisional wound model that enable the testing of a rigid polymer implant. The first modification adds sutures that secure the implant within the wound while limiting its manipulation and ensuring complete coverage by the wound edge. The second modification introduces a second, thicker splint on top of the first splint to create a tenting effect for the wound dressing, thereby protecting the implant.
The major contribution of this protocol is its ability to assess physical implants or tissue engineering and regenerative medicine research. We have successfully used this model to evaluate a polyethylene implant, augmented with an electrospun polymer layer soaked in a protein mixture. Importantly, this approach can be adapted to test a diverse array of scaffolds and can potentially extend its application to organ repair beyond the skin.
Materials and reagents
Reagents
Sodium chloride injection 0.9%, USP (Hospira, NDC, catalog number: 0409 -4888-02)
TerrellTM isoflurane, USP (Piramal Critical Care, NDC, catalog number: 66794-019-25)
Buprenorphine extended release 1.3 mg/mL (Fidelis Animal Health, NDC, catalog number: 86084-100-30)
Laboratory supplies
FisherbrandTM sterile alcohol prep pads (Fisher Scientific, catalog number: 22-363-750)
Prolene 6–0 suture (Ethicon, catalog number: 8617G) (Figure 1iii)
Ethilon 8–0 suture (Ethicon, catalog number: 2808G) (Figure 1iv)
C57BL/6 mice (The Jackson Laboratory, catalog number: 000664)
FlexWrap E-Z Tear® bandage 4 in. vet wrap (Aspen Veterinary Resources, catalog number: 14100122)
TegadermTM film dressing (3M, catalog number: 1626W)
Depilatory cream (Veet gel cream hair remover, catalog number: 3116875)
Triple antibiotic ointment (Padagis, NDC, catalog number: 45802-143-01)
Sterile cotton-tipped applicators (Medline, catalog number: MDS202000H)
LubriFreshTM lubricant eye ointment (Major, NDC, catalog number: 0904-6488-38)
Coach athletic tape (Johnson & Johnson, catalog number: JJ5188)
DuraporeTM surgical tape (3M, catalog number: B00042420)
Cyanoacrylate brush-on glue (Krazy Glue, catalog number: KG92548R)
Model MEDPOR® implant (Stryker, catalog number: 83020)
Betadine swab sticks (Emerson Healthcare, catalog number: 6761815301)
Insulin syringe with needle 1 mL 27G (Becton Dickinson, catalog number: 329412)
Syringe with attached needle 1 mL 25G (Becton Dickinson, catalog number: 309626)
Instant sealing sterilization pouches (Fisher Scientific, catalog number: 01-812-54)
Non-sterile woven gauze sponges (Medline, NON25212H) (Figure 1ii)
Towel drapes (Dynarex, catalog number: 4410)
Disposable dissection board (Mopec, catalog number: 22-444-314) (Figure 1i)
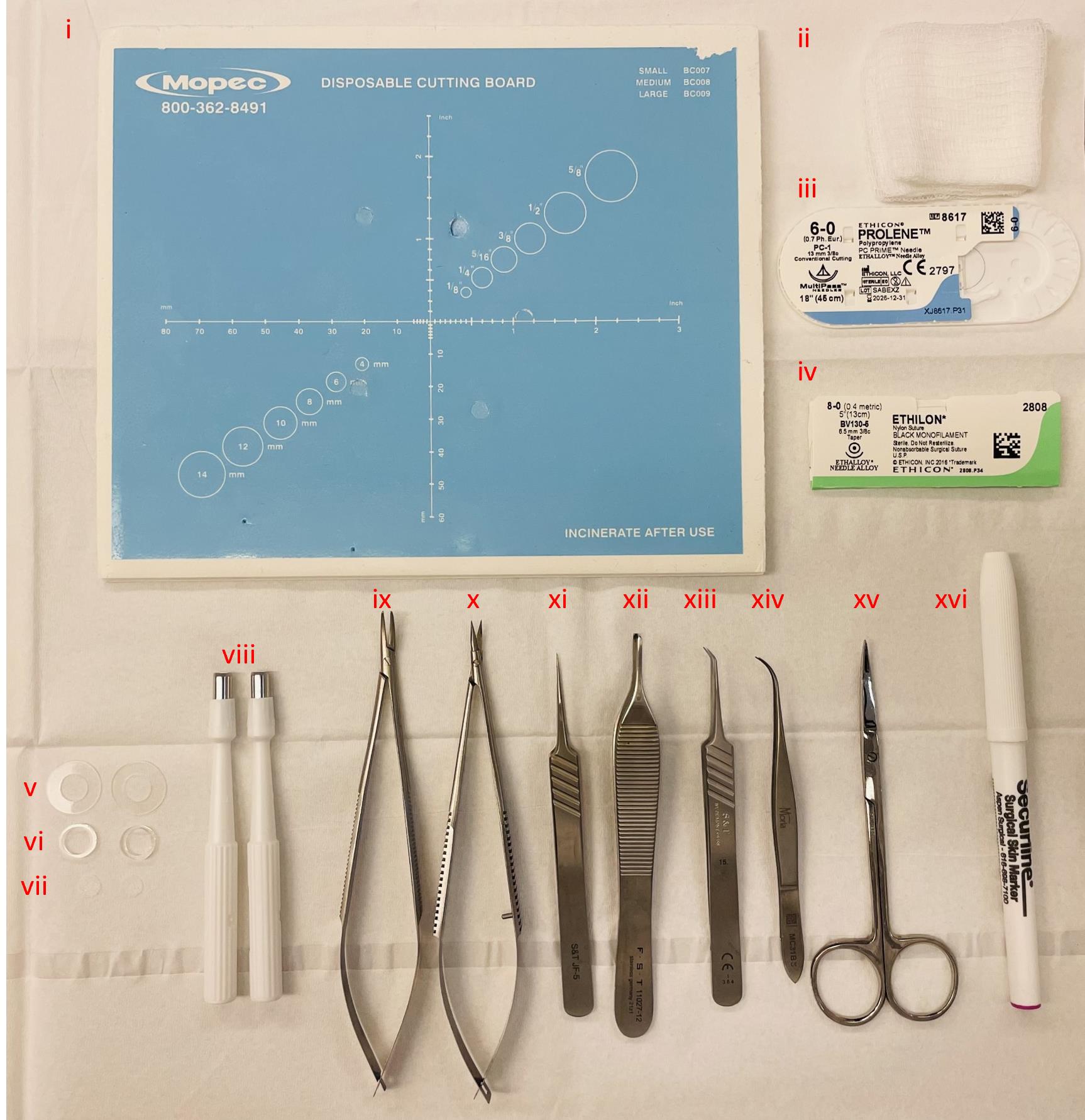
Figure 1. Instruments utilized during surgery. (i) Sterile dissection board, (ii) autoclaved gauze sponges, (iii) 6–0 Prolene suture, (iv) 8–0 Ethilon suture, (v) 0.5 mm thin silicone splints, (vi) 1.6 mm thick silicone splints, (vii) 6 mm diameter model MEDPOR® implants, (viii) 5 mm disposable biopsy punches to create wounds, (ix) needle holder, (x) spring scissors, (xi) straight tip forceps, (xii) Adson forceps, (xiii) angled tip forceps, (xiv) Moria forceps, (xv) fine scissors, and (xvi) sterile surgical marker.
Equipment
Research stereomicroscope system (Olympus, model number: SZX16)
Dino-lite digital microscope (Dino-Lite Digital Microscope, catalog number: AF4915ZTL)
Tuttnauer digital autoclave (Tuttnauer, model number: 2340E-B/L)
Anesthesia system (Veterinary Anesthesia Systems, catalog number: VAS 2001R)
Vetiva® mini trimmer (Wahl, catalog number: 88420)
Premium king-size heating pad (Sunbeam, catalog number: 938-511-000R)
Disposable biopsy punches 5 mm (Acuderm Inc, model: P550) (Figure 1viii)
Disposable biopsy punches 6 mm (Acuderm Inc, model: P625)
Disposable biopsy punches 8 mm (Acuderm Inc, model: P825) (Figure 2Bii)
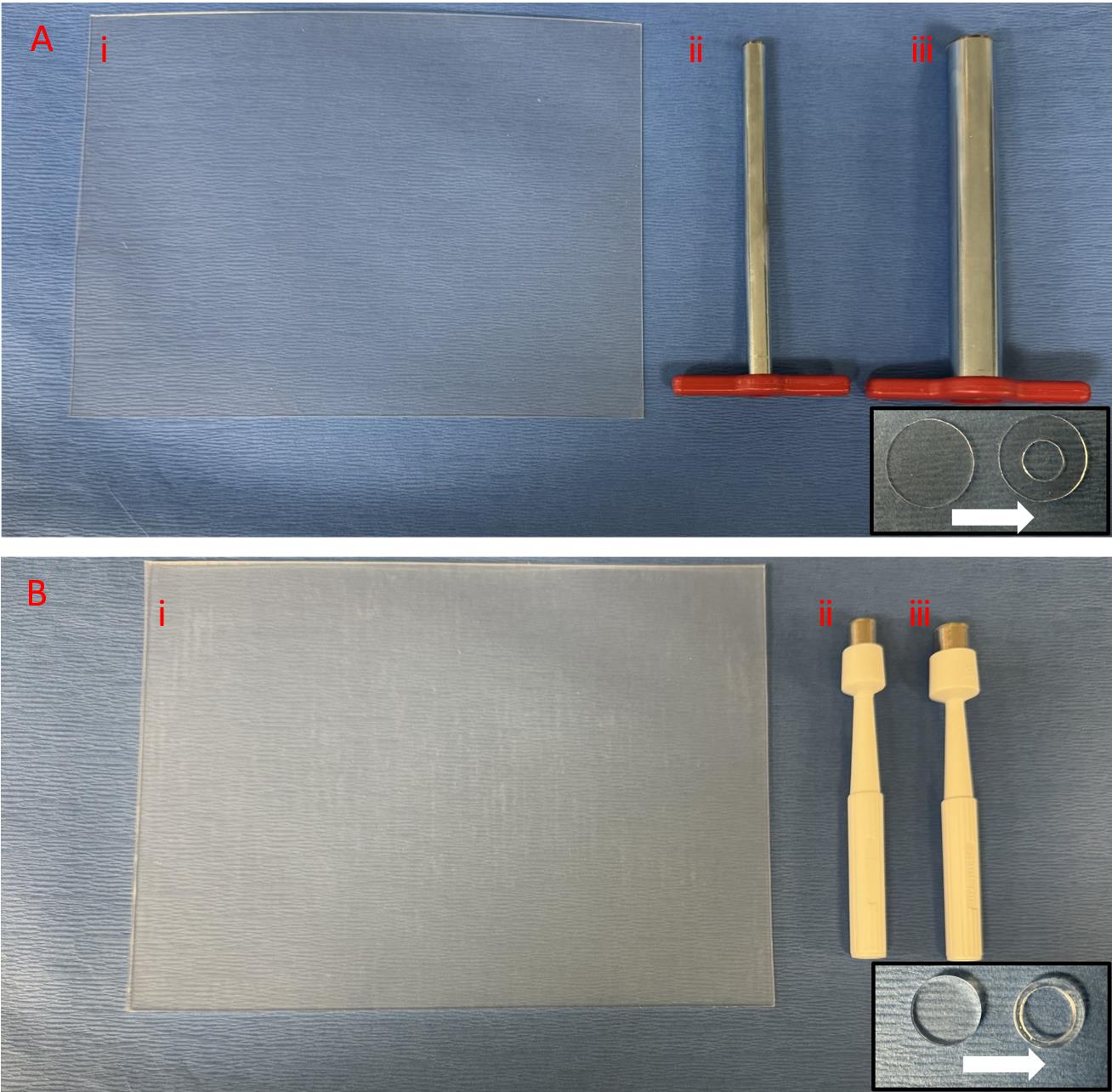
Figure 2. Splint preparation. A. Thin 0.5 mm silicone sheet with 15 mm cork borer to punch the outer circle and 7 mm cork borer to punch the inner circle. B. Thick 1.6 mm silicone sheet with 10 mm punch to punch the outer circle and 8 mm punch to punch the inner circle.Disposable biopsy punches 10 mm (Acuderm Inc, P1050) (Figure 2Biii)
Cork borer set (Humboldt, catalog number: H-9665)
Spring scissors 6 mm cutting edge (Fine Science Tools, catalog number: 15021-15) (Figure 1x)
Adson forceps 1.5 mm tip width (Fine Science Tools, catalog number: 11027-12) (Figure 1xii)
Moria iris forceps 0.5 mm tip width (Fine Science Tools, catalog number: 11373-12) (Figure 1xiv)
S&T needle holder without lock (Fine Science Tools, catalog number: C-14W; 00088-11) (Figure 1ix)
S&T forceps 0.3 × 0.25 mm angled tip (Fine Science Tools, catalog number: 00109-11) (Figure 1xiii)
S&T forceps 0.3 × 0.25 mm straight tip (Fine Science Tools, catalog number: 00108-11) (Figure 1xi)
Fine scissors (Fine Science Tools, catalog number: 14029-10) (Figure 1xv)
Sterile surgical marker (Aspen Surgical Products, catalog number: 1002-00-PDG) (Figure 1xvi)
BambooTM pad (Wacom, catalog number: CTH300U/U0-AX)
Non-adhesive silicone sheet, 0.5 mm (Sigma-Aldrich, Grace Bio-Labs CultureWellTM, GBL664571-5EA) (Figure 2Ai)
Non-adhesive silicone sheet, 1.6 mm (Sigma-Aldrich, Grace Bio-Labs CultureWellTM, GBL664273-5EA) (Figure 2Bi)
Dino-lite adjustable precision mount (Dino-Lite Digital Microscope, catalog number: RK-06A)
Software
DinoCapture 2.0 (Dino-Lite Digital Microscope, dino-lite.com)
ImageJ (National Institutes of Health, imagej.nih.gov)
Excel (Microsoft, microsoft.com)
StatPlus LE (AnalystSoft, analystsoft.com)
Procedure
文章信息
稿件历史记录
提交日期: Oct 1, 2023
接收日期: Mar 11, 2024
在线发布日期: Sep 20, 2024
出版日期: Sep 20, 2024
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Gadalla, D., Kennedy, M. and Lott, D. (2024). Protocol for the Implantation of Scaffolds in a Humanized Mouse Cutaneous Excisional Wound Healing Model. Bio-protocol 14(18): e4974. DOI: 10.21769/BioProtoc.4974.
分类
生物工程 > 生物医学工程
细胞生物学 > 组织分析 > 组织染色
医学
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link













