- EN - English
- CN - 中文
Transient Expression Assay and Microscopic Observation in Kumquat Fruit
金桔果实瞬时表达测定与显微观察
发布: 2024年04月05日第14卷第7期 DOI: 10.21769/BioProtoc.4968 浏览次数: 1738
评审: Wenrong HeXiongjie ZhengMin Cao
Abstract
Citrus fruits encompass a diverse family, including oranges, mandarins, grapefruits, limes, kumquats, lemons, and others. In citrus, Agrobacterium tumefaciens–mediated genetic transformation of Hongkong kumquat (Fortunella hindsii Swingle) has been widely employed for gene function analysis. However, the perennial nature of woody plants results in the generation of transgenic fruits taking several years. Here, we show the procedures of Agrobacterium-mediated transient transformation and live-cell imaging in kumquat (F. crassifolia Swingle) fruit, using the actin filament marker GFP-Lifeact as an example. Fluorescence detection, western blot analysis, and live-cell imaging with confocal microscopy demonstrate the high transformation efficiency and an extended expression window of this system. Overall, Agrobacterium-mediated transient transformation of kumquat fruits provides a rapid and effective method for studying gene function in fruit, enabling the effective observation of diverse cellular processes in fruit biology.
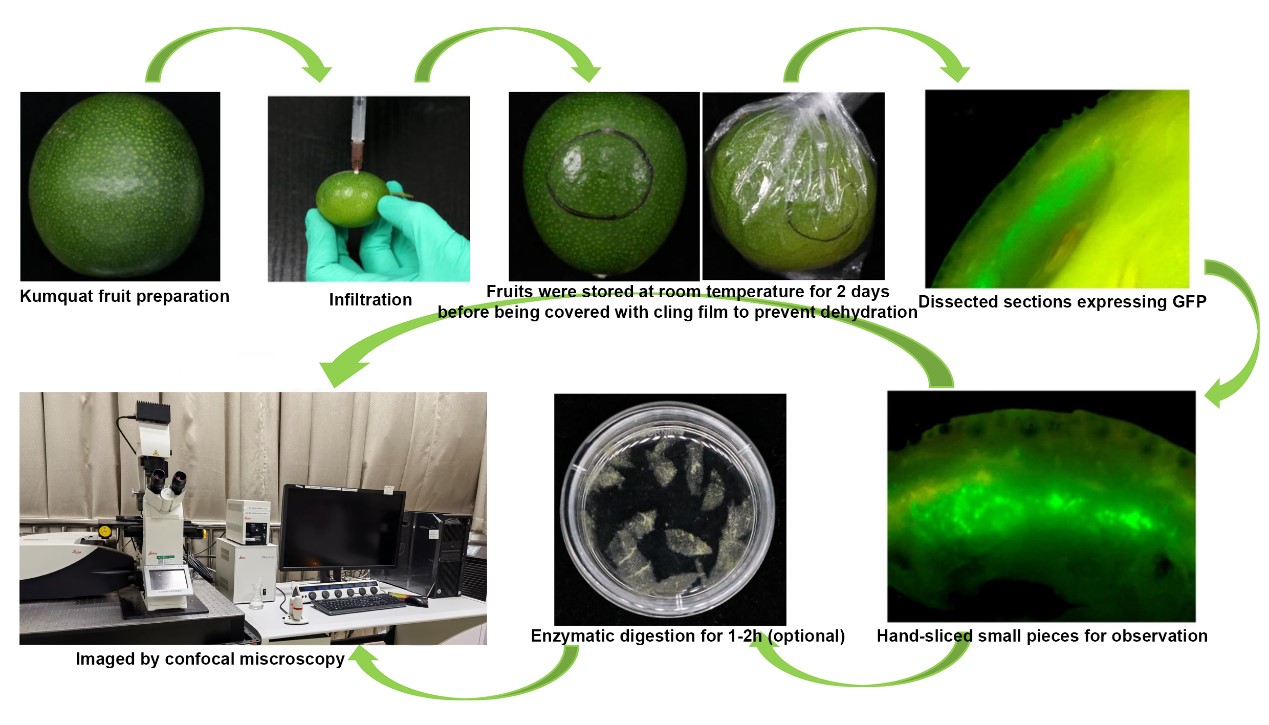
Graphical overview
Background
Citrus, one of the most important fruit crops in the world, is subjected to a variety of environmental stimuli and developmental signals during its growth. Observing the dynamics of organelles may provide new insights into the vital behavior of fruits in response to these developmental and environmental signals. The use of green fluorescent protein (GFP) and its derivatives is a great improvement for cell biological studies, as it can be fused to genes of interest to study their subcellular localization, dynamics, and protein–protein interactions [1]. However, cell structures and subcellular activities from citrus fruits can hardly be observed by light microscopy due to their 3D structure and little transparency. The lack of efficient transformation techniques and the long juvenile phase of citrus plants [2] make the study of cell biology even more difficult. Therefore, developing an effective method for transient transformation of citrus species is important. Agrobacterium-mediated infiltration of tobacco leaves was an early-developed method for transient expression [3] and has been widely used to study cell morphology and dynamics. Subsequently, Agrobacterium-mediated transient expression methods have been developed for a wide range of leaves and fruits including Arabidopsis [4], tomato [5], strawberry [6], apple [7,8], and also citrus fruits [9,10]. Here, the kumquat (Fortunella crassifolia Swingle) fruit has been selected for Agrobacterium-mediated transient expression. It is a major citrus cultivar in southern China, bearing smaller fruits in the Citrus genus compared to pomelo, oranges, and tangerines [11]. It is also characterized by its high sugar content, thin skin, and fewer juice cells, which provides a good environment for infiltration and infection by Agrobacterium [12].
In eukaryotic cells, the cytoskeleton is a network of protein–fiber reticulum consisting of microtubules, microfilaments, and intermediate filaments, which not only plays an important role in maintaining cell morphology, withstanding external forces, and maintaining the orderliness of the internal cell structure, but also participates in many important biological processes. Hence, imaging this network in its native and mobile states is of great importance. Here, using the GFP-Lifeact for actin cytoskeleton as an example, we describe methods for Agrobacterium--mediated transient transformation of citrus fruit and live-cell imaging in citrus fruit. The high expression of fluorescent protein in kumquat fruit, combined with subsequent live cell imaging, provides a method for studying gene function, subcellular localization, and cellular activity in fruit.
Materials and reagents
Biological materials
Kumquat (F. crassifolia Swingle) fruit (collected from Guangxi Institute of Citrus Research, located at Guilin city, Guangxi, China)
Reagents
Actin cytoskeleton marker GFP-Lifeact containing a 35S::GFP cassette as a visual reporter [13]
P19 Agrobacterium
Yeast extract (OXOID, catalog number: LP0021)
Tryptone (OXOID, catalog number: LP0042)
NaCl (HUSHI, catalog number: 10019318, CAS: 7647-14-5)
D-glucose (HUSHI, catalog number: 10010518, CAS: 14431-43-7)
MES (Sigma-Aldrich, catalog number: M8250)
Na3PO4 (HUSHI, catalog number: 20040928, CAS: 7601-54-9)
Acetosyringone (Sigma-Aldrich, catalog number: D134406)
GV3101 Chemically Competent Cell (Shanghai Weidi Biotechnology, catalog number: AC1001)
Antibiotics: Kanamycin (Kan) (Genview, catalog number: AK177-10G) and Rifampicin (Rif) (Genview, catalog number: AR280-1G)
Antibodies: Polyclonal mouse antibody against GFP (Biorbyt, catalog number: orb688437) as the primary antibody and goat anti-mouse HRP (Biorbyt, catalog number: orb670233) as the secondary antibody
Dimethyl sulfoxide (DMSO) (MACKLIN, catalog number: C13178602)
Mannitol (Solarbio, catalog number: M8141)
KCl (Rhawn, catalog number: R051965)
CaCl2 (MACKLIN, catalog number: C832203)
BSA (MACKLIN, catalog number: B928042)
Cellulase R10 (Yakult, catalog number: 220908-02)
Sodium hypochlorite (MACKLIN, catalog number: S935360)
Solutions
Luria Broth (LB) medium (see Recipes)
Infiltration buffer (see Recipes)
Enzymatic solution (see Recipes)
Antibiotics (see Recipes)
Recipes
LB media
10 g of tryptone, 10 g of NaCl, 5 g of yeast extract; make up to 1 L with dH 2O, following autoclave sterilization.
Infiltration buffer
Infiltration medium stock solutions:
500 mM MES: 4.88 g of MES in 50 mL of dH2O. Store at 4 °C.
20 mM Na3PO4: 0.17 g of Na3PO4 in 50 mL of dH2O. Store at 4 °C.
1 M Acetosyringone (3’,5’-dimethoxy-4’-hydroxyacetophenone): 0.196 g of Acetosyringone in 1 mL of DMSO. Divide into single-use aliquots and store at -20 °C.
50 mL infiltration buffer
250 mg of D-glucose, 5 mL of MES stock solution, 5 mL of Na3PO 4 stock solution, 5 μL of Acetosyringone stock solution; make up to 50 mL with dH2O.
Enzymatic solution
Enzymatic solution stock solutions:
0.8 M mannitol stock: 7.29 g of mannitol in 50 mL of dH2O.
2 M KCl stock: 7.455 g of KCl in 50 mL of dH2O.
0.2 M MES stock: 1.952 g of MES in 50 mL of dH2O. Store at 4 °C.
1 M CaCl2 stock: 1.11 g of CaCl2 in 10 mL of dH2 O.
10% BSA stock: 0.1 g of BSA in 1 mL of dH2O.
10 mL enzymatic solution: 0.15 g of cellulase R10, 0.04 g of macerozyme R1, 5 mL of 0.8 M mannitol stock, 100 μL of 2 M KCl stock, and 1 mL of 0.2 M MES (pH 5.7) stock. Heat solution in heat block or oven set to 55 °C (maximum) for 10 min to help enzymes dissolve (or place in 55 °C water) and then cool to room temperature (RT). Next, add 100 μL of 1 M CaCl 2 stock, 100 μL of 10% BSA stock, and dH2O up to 10 mL. Sterilize by filtration (0.45 μm).
Antibiotics
50 mg/L Rif
50 mg/L Kan
Laboratory supplies
Tips (Axygen, catalog number: AXYT1000B)
1 mL syringe with needle (GEMTIER, catalog number: 0.45X16 RW LB)
Glass microscope slides, coverslips (Corning, catalog number: CLS294875X25, CLS285522), and Vectashield (Vectorlabs, catalog number: H-1000) for microscopy
Test tubes (Eppendorf, catalog number: EP0030122178) and flask (Corning, catalog number: CLS4444250) for culturing Agrobacterium
Fine forceps and razor blades (Artis Tweezer, catalog number: Z742671; smartSlicer, catalog number: Z740503)
Marker pen (STATMARK, catalog number: Z648191)
Tray (PureSolv, catalog number: Z681687) and Falcon tube cap (Corning, catalog number: 352070) for placing fruit
Petri dishes (60 mm, optional, for enzymatic digestion of protoplasts) (Nalgene, catalog number: TMO5921-0060)
Pipette (Eppendorf, catalog number: GN686271)
Rubber gloves (Microflex, catalog number: Z265179)
Bibulous paper (for uptaking the extravasated Agrobacterium)
50 mL Falcon tubes (Corning, catalog number: 352070)
Equipment
Shaking incubator (Radobio, model: Stab M1T) used for the growth of Agrobacterium at 28 °C
Centrifuge (Eppendorf, model: 5424)
Clean workbench (AIRTECH, model: SW-CJ-2FD)
Fluorescence dissecting stereomicroscope (Olympus, model: SZX7)
Confocal microscope (Leica, model: SP8)
Nanodrop spectrophotometer (or equivalent) to determine optical density of bacterial culture
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY license (https://creativecommons.org/licenses/by/4.0/).
如何引用
Gong, J. and Sun, X. (2024). Transient Expression Assay and Microscopic Observation in Kumquat Fruit. Bio-protocol 14(7): e4968. DOI: 10.21769/BioProtoc.4968.
分类
植物科学 > 植物细胞生物学 > 细胞成像
植物科学 > 植物分子生物学 > 蛋白质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link