- EN - English
- CN - 中文
Purification of Photorhabdus Virulence Cassette (PVC) Protein Complexes from Escherichia coli for Artificial Translocation of Heterologous Cargo Proteins
从大肠杆菌中纯化发光杆菌毒力盒(PVC)蛋白复合物,用于异源货物蛋白的人工转运
(*contributed equally to this work) 发布: 2024年04月05日第14卷第7期 DOI: 10.21769/BioProtoc.4966 浏览次数: 2231
评审: David A. CisnerosBhanu JagilinkiJoyce ChiuGuillaume Lenoir

相关实验方案

Cluster FLISA——用于比较不同细胞系蛋白表达效率及蛋白亚基聚集状态的方法
Sabrina Brockmöller and Lara Maria Molitor
2025年11月05日 1209 阅读
Abstract
Contractile injection systems (CISs), one of the most important bacterial secretion systems that transport substrates across the membrane, are a collection of diverse but evolutionarily related macromolecular devices. Numerous effector proteins can be loaded and injected by this secretion complex to their specific destinations. One group of CISs called extracellular CIS (eCIS) has been proposed as secretory molecules that can be released from the bacterial cytoplasm and attack neighboring target cells from the extracellular environment. This makes them a potential delivery vector for the transportation of various cargos without the inclusion of bacterial cells, which might elicit certain immunological responses from hosts. We have demonstrated that the Photorhabdus virulence cassette (PVC), which is a typical eCIS, could be applied as an ideal vector for the translocation of proteinaceous cargos with different physical or chemical properties. Here, we describe the in-depth purification protocol of this mega complex from Escherichia coli. The protocol provided is a simpler, faster, and more productive way of generating the eCIS complexes than available methodologies reported previously, which can facilitate the subsequent applications of these nanodevices and other eCIS in different backgrounds.
Keywords: Contractile injection system (缩收式注射系统)Background
Typical contractile injection systems (CISs) have been studied for decades for their structure, assembly, and mechanism. They can take part in multiple interactions between microorganisms and hosts by delivering nucleic acids and protein substrates using contractile sheath–tube assemblies, such as the contractile tails of bacteriophage T4, P2, and Mu [1]. The type VI secretion system, which spans both the inner and outer membranes of Gram-negative bacteria and transports substrates from the cytoplasm, is a well-studied example of a contractile apparatus [2]. Extracellular CIS (eCISs), found in both prokaryotes and archaea, such as Photorhabdus virulence cassette (PVC), the antifeeding prophage [3], and the metamorphosis-associated contractile structure [4], have also been studied extensively in recent years.
Although the species from the Photorhabdus genus are generally considered pathogens infecting insects, P. asymbiotica has been reported to infect humans in the United States and Australia [5]. Five PVC clusters have been identified in the genome of P. asymbiotica, all phylogenetically related to the tail of T4 bacteriophage. PVC could translocate toxins into eukaryotic cells [6], which means PVC is different from T4 phage or R-type pyocins, which can only target prokaryotic cells. Moreover, a group of N-terminal signal peptides are identified within the PVC effectors to facilitate the cargo loading process [7]. Taking into consideration that PVC complexes could be released outside the bacterial cells for function, this apparatus would be an ideal model for manipulation as a potent delivery vector in precision medicine [8].
The purification workflow of PVC can be divided into four steps: bacterial collection, cell rupture, cell debris removal, and protein extraction. Centrifugation and ultracentrifugation are the primary methods for bacterial collection, cell debris removal, and protein extraction. As for the cell rupture, chemical and biological methods are applied to break the cell membrane and release the PVC protein complexes. This protocol has been used several times to purify PVC complexes for structural and functional characterization experiments, demonstrating the effectiveness of this method.
Materials and reagents
15 mL centrifuge tubes (Corning, catalog number: 430791)
50 mL centrifuge tubes (Corning, catalog number: 430829)
500 mL triangular culture flask (Corning, catalog number: 431145)
1.5 mL microtubes (Axygen, catalog number: MCT-150-C-S)
Sterile syringe filter (Millipore, catalog number: SLGVR33RB)
500 mL centrifuge bottle (Nalgene, catalog number: 3120-0500)
50 mL round bottom centrifuge tubes (Biosharp, catalog number: BS-500-MC)
13.2 mL centrifuge tubes (Beckman Coulter, catalog number: 344059)
Escherichia coli strain EPI300 (Lucigen, catalog number: EC300110)
Plasmids pCNM3, pBR60, and pBBRN Pnf N50-TkTcsT, which were used previously [4,7] (Figure 1)
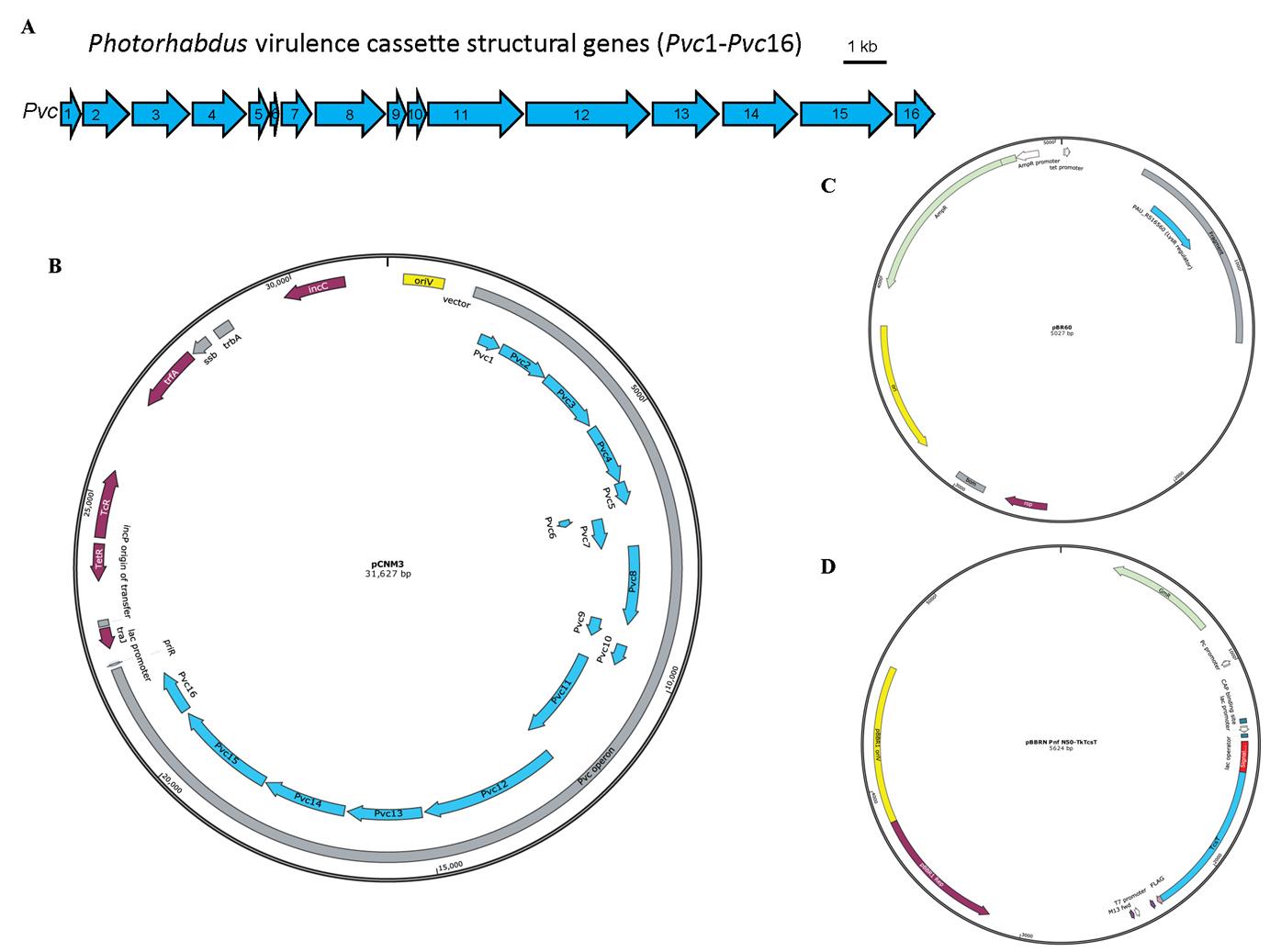
Figure 1. Schematic illustration of the Photorhabdus virulence cassette (PVC) locus and the map of plasmids used for PVC complex and cargo protein production. A. The 16 PVC structural genes (annotated Pvc1–Pvc16, shown in blue) involved in protein complex production are encoded consecutively within the P. asymbiotica genome. B–D. The plasmids pCNM3 (B), pBR60 (C), and pBBRN Pnf N50-TkTcsT (D) were constructed by our lab and the maps were generated using SnapGene. pCNM3 was constructed by ligating the complete PVC operon into pRK404 vector for constitutive expression under their own promoters [9]. pBR60 was created by ligating the DNA fragment containing the PAU_RS16560 (LysR regulator gene, shown in blue) into pBR322 plasmid (BamHI-SalI insertion) for constitutive expression of the regulator gene under its own promoter. pBBRN Pnf N50-TkTcsT was a plasmid used for cargo production. This plasmid was created by inserting the open reading frame of the toxic TkTcsT protein (shown in blue) from Trichosanthes kirilowii into a broad host range vector pBBR1MCS5, with a PVC signal peptide sequence (shown in red) fused at the N-terminus of the TkTcsT protein. This allows the production of signal peptide–fused proteins in E. coli.LB broth, powder (Solarbio, catalog number: L1010)
Phosphate-buffered saline (PBS) (Gibco, catalog number: C10010500BT)
Tetracycline HCl (Solarbio, catalog number: T8180)
Ampicillin, sodium salt (Solarbio, catalog number: A8180)
Gentamycin sulfate (Solarbio, catalog number: G8170)
Anhydrous ethanol (Beijing Chemical Works, catalog number: C320080)
TBS tablets (VWR, catalog number: K859-100TABS)
Triton X-100 solution (Sigma, catalog number: X-100)
Protease inhibitor cocktail (MCE, catalog number: HY-K0010)
Lysozyme (Sigma, catalog number: L6876)
DNase I (MCE, catalog number: HY-108882)
Sodium chloride (Solarbio, catalog number: S8210)
Magnesium chloride hexahydrate (Solarbio, catalog number: M8161)
Solution endotoxin erasol (TianDZ, catalog number: 60607-25)
Solutions
LB broth medium (see Recipes)
10 mg/mL tetracycline in solution (see Recipes)
100 mg/mL ampicillin in solution (see Recipes)
50 mg/mL gentamycin in solution (see Recipes)
5 mg/mL NaCl (see Recipes)
2 M MgCl2 (see Recipes)
20 mg/mL lysozyme (see Recipes)
5 mg/mL DNase I (see Recipes)
P buffer (see Recipes)
Recipes
LB broth medium
Add 25 g of LB broth powder to 1,000 mL of distilled water. Sterilize using a high-pressure steam sterilization pot at 121 °C for 15 min.
10 mg/mL tetracycline in solution
Add 0.5 g of tetracycline HCl to 50 mL of anhydrous ethanol. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
100 mg/mL ampicillin in solution
Add 1 g of ampicillin to 10 mL of distilled water. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
50 mg/mL gentamycin in solution
Add 1 g of gentamycin sulfate to 20 mL of distilled water. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
5 mg/mL NaCl
Dissolve 1 g of sodium chloride in 200 mL of distilled water. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
2 M MgCl2·6H2O
Dissolve 406 g of magnesium chloride hexahydrate in 800 mL of distilled water and adjust volume to 1 L. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
20 mg/mL lysozyme
Dissolve 1 g of lysozyme in 50 mL of distilled water. Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
5 mg/mL DNase I
Dissolve a bottle of DNase I (100 mg) in 20 mL of NaCl solution (5 mg/mL). Filter sterilize with a 0.22 µm pore size hydrophilic PVDF membrane.
P buffer
Take 50 mL of distilled water into a 50 mL centrifuge tube and add a piece of TBS tablet. Divide the solution into two 50 mL centrifuge tubes equally after the TBS tablet is completely dissolved.
Add 2.5 mL of 10% Triton X-100 solution, 500 μL of 20 mg/mL lysozyme, 500 μL of 5 mg/mL DNase I, 125 μL of 2 M MgCl2, and 500 μL of protease inhibitor cocktail to each tube. Add distilled water to fill 50 mL.
Notes:
P buffer should be freshly prepared.
TBS tablets could cause skin and eye irritation; avoid breathing dust, fumes, gas, mist, vapors, or spray.
Triton X-100 solution could cause serious eye damage; wear protection when using it.
The final concentration of lysozyme and DNase I are 200 µg/mL and 50 µg/mL in P buffer, respectively. Triton X-100 and MgCl2 are 50 mg/mL, 0.5%, and 5 mM, respectively.
Equipment
Innova44 Incubator Shaker Series (NBS, model: SHA1104)
Biological safety cabinet (Shanghai Lishen Scientific Equipment Co, model: Hfsafe 1200LC)
Milli-Q (Millipore, catalog number: IX 70XX)
High-pressure steam sterilization pot (YAMATO, model: SM830)
High-speed refrigerated centrifuge (Eppendorf, model: 5424 R)
Ultracentrifuge (Beckman, model: Optima L-100 XP)
-80 °C ultra-low temperature freezer (Haier, model: DW-86L728J)
Thermostatic water bath (GFL, model: GFL1002)
High-speed refrigerated centrifuge (Hitachi, model: CR22G)
Ice machine (Panasonic, model: SIM-F140BDL)
NanoDrop 1000 spectrophotometer
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Wang, Y., Zhang, X., Feng, X., Wang, X., Jin, Q. and Jiang, F. (2024). Purification of Photorhabdus Virulence Cassette (PVC) Protein Complexes from Escherichia coli for Artificial Translocation of Heterologous Cargo Proteins. Bio-protocol 14(7): e4966. DOI: 10.21769/BioProtoc.4966.
- Jiang, F., Shen, J., Cheng, J., Wang, X., Yang, J., Li, N., Gao, N. and Jin, Q. (2022). N-terminal signal peptides facilitate the engineering of PVC complex as a potent protein delivery system. Sci. Adv. 8(17): eabm2343.
分类
微生物学 > 异源表达系统 > 大肠杆菌
生物化学 > 蛋白质 > 表达
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link











