- EN - English
- CN - 中文
Method for Large-scale Production of hIPSC Spheroids
大规模生产hIPSC球体的方法
发布: 2024年04月05日第14卷第7期 DOI: 10.21769/BioProtoc.4965 浏览次数: 1944
评审: Alessandro DidonnaLionel SchiavolinAnonymous reviewer(s)
Abstract
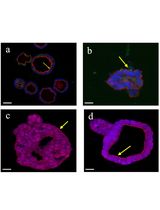
Stem cell spheroids are rapidly becoming essential tools for a diverse array of applications ranging from tissue engineering to 3D cell models and fundamental biology. Given the increasing prominence of biotechnology, there is a pressing need to develop more accessible, efficient, and reproducible methods for producing these models. Various techniques such as hanging drop, rotating wall vessel, magnetic levitation, or microfluidics have been employed to generate spheroids. However, none of these methods facilitate the easy and efficient production of a large number of spheroids using a standard 6-well plate. Here, we present a novel method based on pellet culture (utilizing U-shaped microstructures) using a silicon mold produced through 3D printing, along with a detailed and illustrated manufacturing protocol. This technique enables the rapid production of reproducible and controlled spheroids (for 1 × 106 cells, spheroids = 130 ± 10 μm) from human induced pluripotent stem cells (hIPSCs) within a short time frame (24 h). Importantly, the method allows the production of large quantities (2 × 104 spheroids for 1 × 106 cells) in an accessible and cost-effective manner, thanks to the use of a reusable mold. The protocols outlined herein are easily implementable, and all the necessary files for the method replication are freely available.
Key features
• Provision of 3D mold files (STL) to produce silicone induction device of spheroids using 3D printing.
• Cost-effective, reusable, and autoclavable device capable of generating up to 1.2× 104 spheroids of tunable diameters in a 6-well plate.
• Spheroids induction with multiple hIPSC cell lines.
• Robust and reproducible production method suitable for routine laboratory use.
Graphical overview
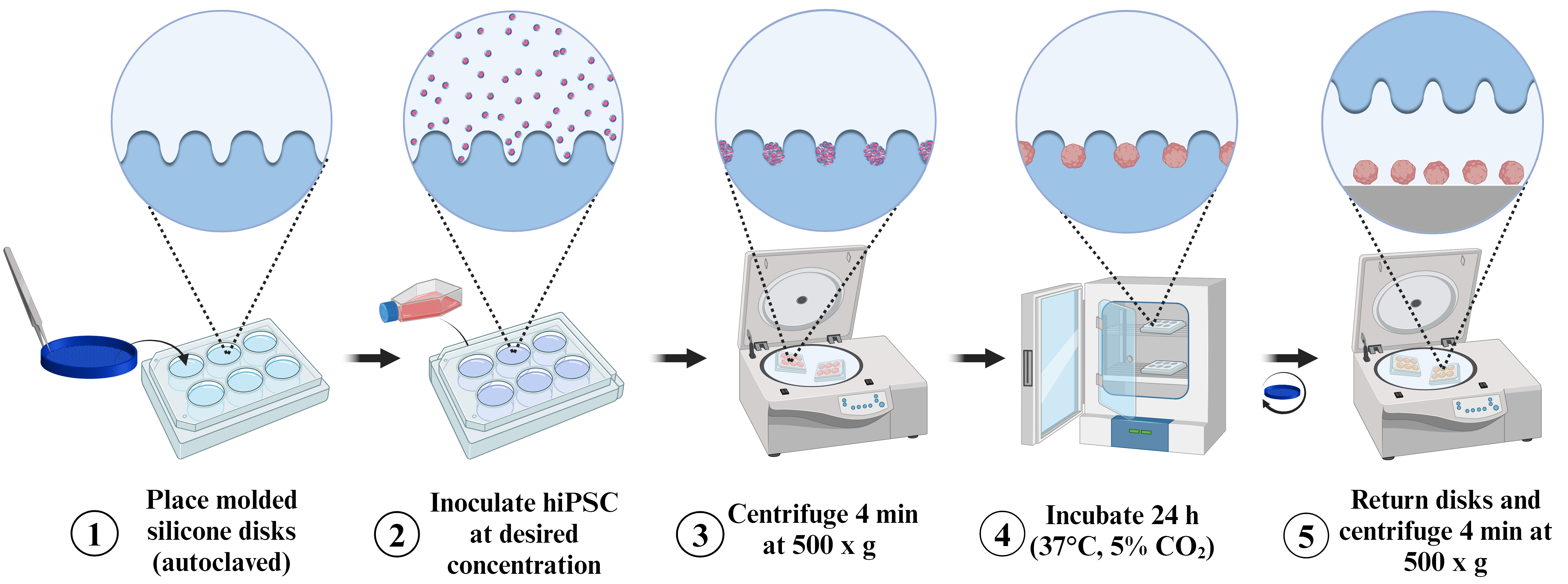
Spheroid induction process following the pellet method on molded silicon discs
Background
Stem cell spheroids ideally mimic the physiological 3D environment of tissues, making them increasingly indispensable in tissue engineering [1]. Since the inception of cellular aggregates in the 1930s, spheroids have become a model of choice across various fields of fundamental biology. Their applications span from drug testing to disease modeling, encompassing oncology and regenerative medicine [2,3]. Despite the growing use of spheroids in the past two decades, the demand for spheroids as a raw material remains substantial [4]. This development has given rise to numerous spheroid production methods, tailored to specific applications.
Concurrently, the use of human induced stem cells (hiPSCs) has surged since their discovery in 2007 [5]. This model, based on the reprogramming of unipotent cells into pluripotent ones, proves valuable from practical and ethical perspectives, offering an excellent substitute for embryonic stem cells. Today, hIPSCs play a pivotal role in both basic research and applied biology [6,7]. Simultaneously, spheroids represent a highly promising tool for both tissue engineering [8] and the study of fundamental biological phenomena [9] and have been recently used to assess the impact of the viscoelastic properties of the 3D environment on their growth [10]. The most employed techniques to form spheroids from stem cells, including hiPSCs, are rotary cell culture, hanging drop, cell culture in non-adhesive hydrogel, pellet method, and microfluidics [11]. However, these methods share drawbacks related to low productivity, time inefficiency, high cost, and very limited reusability.
The technique presented here is built upon the pellet method (U-shaped), offering a compromise between existing methods. Optimized for rapid and cost-effective production of well-defined spheroids from hiPSCs, this solution is easily transferable for integration into a routine production pipeline. Exhibiting the ability to tune the diameter of spheroids (50–150 μm) by modulating the number of cells used (from 1.0 × 105 to 1.5 × 106), its adaptability extends to other cell lines, demonstrating robust aggregation capabilities.
Materials and reagents
Biological materials
hiPSCs AG08C5 cell line (European hPSCreg as PGNMi001-A) ( https://hpscreg.eu/cell-line/PGNMi001-A) [12]
Healthy Control Human iPSC Line, Female, SCTi003-A (STEMCELL, catalog number: 200-0511)
Reagents
Maintenance culture media mTeSRTM Plus kit, cGMP (STEMCELL, catalog number: 100-0276)
Vitronectin XFTM (STEMCELL, catalog number: 100-0763)
TrypLETM (Thermo Fisher, catalog number: 12604013)
Y-27632 (Dihydrochloride), RHO/ROCK pathway inhibitor (STEMCELL, catalog number: 72302)
Fetal bovine serum (FBS) (Sigma Aldrich, catalog number:341506)
Gibco Dulbecco’s phosphate buffered saline (DPBS) (Thermo Scientific, catalog number: 14190)
Soap (e.g., Enzypin, Distrimed, catalog number: 201261)
Ethanol 70% (e.g., Fisher Scientific, catalog number: 16320638)
Solutions
hiPSC spheroids induction medium (see Recipes)
TrypLETM inhibition medium (see Recipes)
Recipes
hiPSC spheroids induction medium
Composition Final concentration Volume mTeSRTM Plus - 50 mL Y-27632 (10 mM) 10 μM 50 μL TrypLETM inhibition medium
Composition Final concentration Volume mTeSRTM Plus - 9.8 mL FBS 2% 200 μL The solutions should be freshly prepared immediately before each experiment, with volumes adjusted according to the specific requirements. Prior to contact with the cells, these solutions must be prewarmed to 37 °C. Antibiotics can be supplemented to the medium according to your culture protocol.
Laboratory supplies
P1,000 pipette (e.g., Pipetman 100–1,000 µL, Gilson, catalog number: F144059M).
Tips 1,000 µL, (e.g., AmpliPur Expert Tips 100–1,000 µL, Gilson, catalog number: F174401)
Acrylic resin (Acrylate-like) (e.g., Stratasys, model: Vero ClearTM )
Silicone (e.g., Elkem Silicones, model: BLUESILTM RTV 3503)
Disposable autoclave bag (Sigma-Aldrich, catalog number: Z692212)
6-well plate, round (Thermo Fischer, catalog number: 140675)
Falcon 15 mL centrifuge tubes, PET, conical bottom w/plug seal cap (Sigma-Aldrich, catalog number: CLS430055)
Falcon 50 mL centrifuge tubes, PET, conical bottom w/plug seal cap (Sigma-Aldrich, catalog number: CLS4558)
Petri dish B60 (e.g., Corning, catalog number: BP53-03)
Tweezer (e.g., EMS 96, Sigma Aldrich, catalog number: 932922)
Disposable hemocytometer (e.g., Millicell®, Sigma-Aldrich, catalog number: MDH-2N1-50PK)
Positive displacement pipette (e.g., Microman 100–1,000 µL, Gilson, catalog number: FD10006)
Capillary pistons (e.g., CP1,000ST 2 × 91 TIPACK, Gilson, catalog number: F148180)
Microtube Eppendorf 2 mL (Dutscher, catalog number: 033297)
Equipment
Inkjet 3D printer (e.g., Stratasys, model: Objects 30 pro)
Autoclave (e.g., Sigma-Aldrich, model: BioCLAVETM mini digital autoclave, catalog number: Z680109)
Centrifuge (e.g., Sigma Aldrich, catalog number: C166500)
CO2 incubator (e.g., MEMMERT, catalog number: I227880)
Vacuum drying oven (e.g., Goldbrunn, model: 450)
Fluid aspiration systems (e.g., BVC control, Vaccubrand, catalog number: 20727200)
6 well-plate rotor (e.g., Eppendorf A-4-81 Rotor, Marshall Scientific, catalog number: EP-WPB)
Software and datasets
MATLAB (2022b, September 20, 2022)
Prism v9.0 (GraphPad, October 27, 2020)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Lemarié, L., Courtial, E. J. and Sohier, J. (2024). Method for Large-scale Production of hIPSC Spheroids. Bio-protocol 14(7): e4965. DOI: 10.21769/BioProtoc.4965.
分类
生物工程 > 生物医学工程
干细胞 > 类器官培养
细胞生物学 > 细胞分离和培养 > 3D细胞培养
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link