- EN - English
- CN - 中文
Phosphoproteomic Analysis and Organotypic Cultures for the Study of Signaling Pathways
用于信号通路研究的磷酸化蛋白质组分析和器官型培养
(*contributed equally to this work) 发布: 2024年02月20日第14卷第4期 DOI: 10.21769/BioProtoc.4941 浏览次数: 2681
评审: Philipp WörsdörferHsih-Yin TanAnonymous reviewer(s)
Abstract
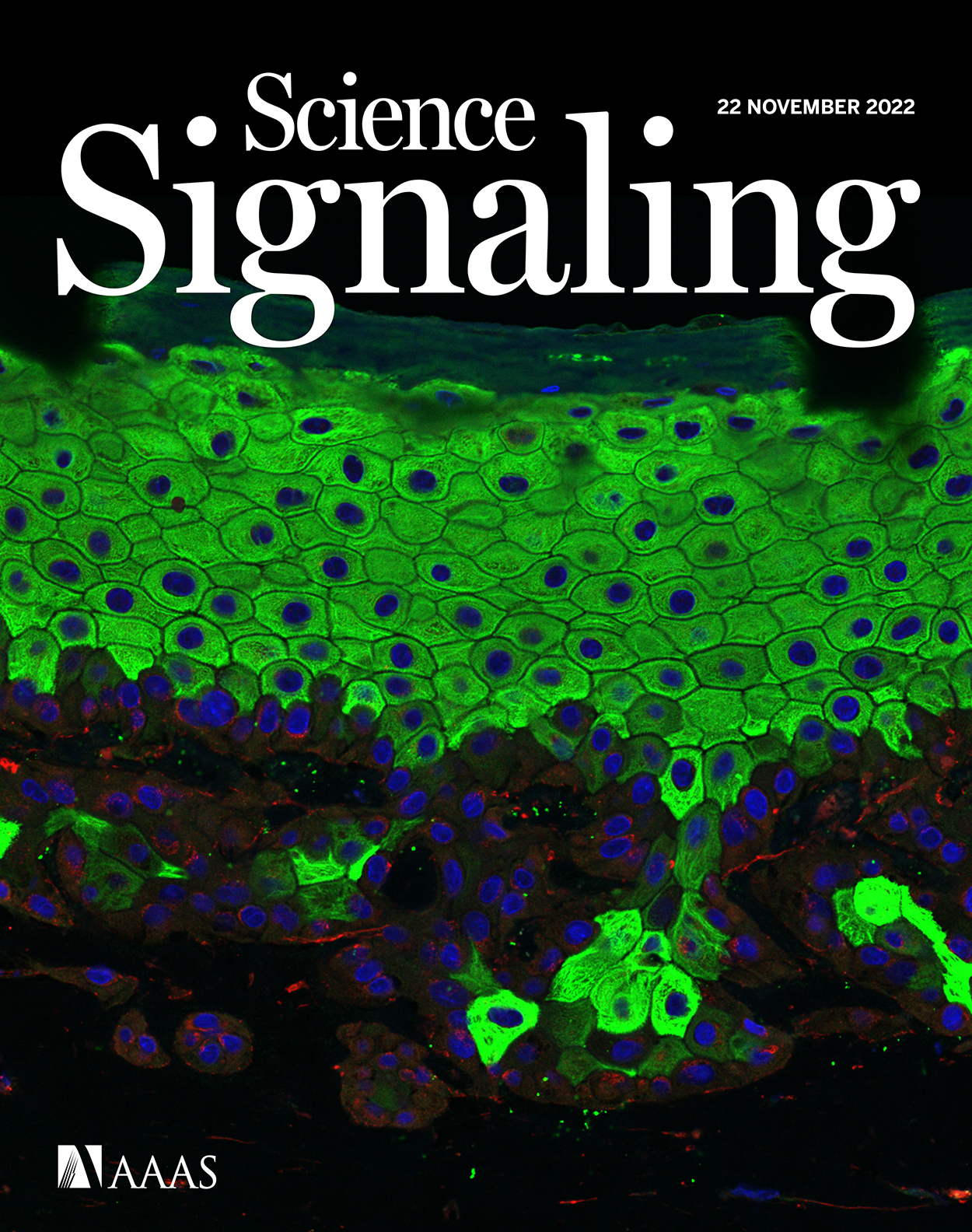
Signaling pathways are involved in key cellular functions from embryonic development to pathological conditions, with a pivotal role in tissue homeostasis and transformation. Although most signaling pathways have been intensively examined, most studies have been carried out in murine models or simple cell culture. We describe the dissection of the TGF-β signaling pathway in human tissue using CRISPR-Cas9 genetically engineered human keratinocytes (N/TERT-1) in a 3D organotypic skin model combined with quantitative proteomics and phosphoproteomics mass spectrometry. The use of human 3D organotypic cultures and genetic engineering combined with quantitative proteomics and phosphoproteomics is a powerful tool providing insight into signaling pathways in a human setting. The methods are applicable to other gene targets and 3D cell and tissue models.
Key features
• 3D organotypic models with genetically engineered human cells.
• In-depth quantitative proteomics and phosphoproteomics in 2D cell culture.
• Careful handling of cell cultures is critical for the successful formation of theorganotypic cultures.
• For complete details on the use of this protocol, please refer to Ye et al. 2022.
Keywords: Organotypic model (器官模型)Background
Signaling pathways are crucial regulators of cell growth and differentiation, playing significant roles in tissue formation and transformation; in many cases, they have been studied in simple 2D cell cultures or in animal studies. While many of the observations from murine models are expected to be applicable to human tissue, there are some inherent difficulties in translating these findings to a human context [1]. Consequently, it is important to use human cell and tissue systems to better understand and translate the functions of different signaling pathways into a human setting. We have previously integrated precise genetic engineering of the human N/TERT-1 and other keratinocyte cell lines to create a genetically traceable starting point to study individual gene products for the formation and transformation of fully differentiated human epidermal tissue [2–4]. The epidermis is a self-renewing, stratified tissue composed of proliferating basal cells and non-proliferating suprabasal cells [5]. Their properties are influenced by the underlying extracellular matrix (ECM) and dermal fibroblasts via intense bidirectional crosstalk mediated by secreted factors [6,7]. This creates the possibility to study both endogenous changes in cells and crosstalk between cell types in a controlled manner [2–4,8]. Therefore, in vitro human skin development is a relevant model to interrogate the various functions of signaling pathways, particularly when combined with proteomics and phosphoproteomics.
Quantitative liquid chromatography–mass spectrometry (LC–MS)-based proteomics and phosphoproteomics have emerged as the preferred methods to study cell signaling [9]. Isobaric labeling methods, like tandem mass tags (TMT), provide relative quantitation of identified proteins and phosphopeptides in multiple samples [10]. Combining the use of genetic engineering, proteomics, and phosphoproteomics with 3D organotypic human tissue models is an important tool in examining signaling pathways, as showed by our group [11]. In that study, we showed the potential different roles between Smad4-dependent and Smad4-independent TGF-β pathways and their role in diverse cellular behaviors such as human tissue homeostasis.
Taken together, these methods can be used as tools for characterizing signaling in human tissue formation and homeostasis. The described protocol details the creation of 3D organotypic cultures (protocol: organotypic tissue culture) that imitate in vivo tissue and its use to examine signaling pathways. Post incubation, the cultures are processed for histology or other analyses. In combination, parallel proteomics and phosphoproteomics analyses (protocol: Proteomics) of 2D cellular cultures, including cell lysis, protein digestion, phosphopeptide enrichment, and LC–MS analysis, allow for a detailed molecular investigation of the affected signaling pathways. The method provides novel insight into cell signaling, relevant for drug development and the understanding of disease pathology. The method can furthermore be used for drug testing and toxicology studies and minimizes the use of animal models.
Materials and reagents
Biological materials
For organotypic tissue culture
MRC-5 (ATCC, catalog number: CCL-171)
N/TERT-1 (James G. Rheinwald, Harvard Institute of Medicine)
For proteomics
Amine (NH2) functional microparticles, MagReSyn® Amine [ReSyn Biosciences (Pty) Ltd., South Africa]
Hydroxyl terminated beads, MagReSyn® Hydroxyl [ReSyn Biosciences (Pty) Ltd., South Africa]
Magnetic microparticles with chelated Ti4 + metal ions for highly specific phosphopeptide enrichment, MagReSyn® Ti-IMAC HP [ReSyn Biosciences (Pty) Ltd., South Africa]
Reagents
For organotypic tissue culture
Penicillin/streptomycin (Thermo Fisher, Gibco, catalog number: 151401)
DMEM (Thermo Fisher, Gibco, catalog number: 41966029)
Keratinocyte-SFM (Thermo Fisher, Gibco, catalog number: 17005034)
TrypLE (Thermo Fisher, Gibco, catalog number: 12605028)
Bovine pituitary extract (BPE) (Thermo Fisher, Gibco, catalog number: 13028014)
Fetal calf serum (FCS) (HyClone, catalog number: SH30071.03HI)
EGF (Thermo Fisher, Gibco, catalog number: PHG0314)
Insulin (Sigma-Aldrich, catalog number: 11070-73-8)
Hydrocortisone (Sigma-Aldrich, catalog number: H0888)
Triiodothyronine (T3) (Sigma-Aldrich, catalog number: T2752)
L-Glutamine (Thermo Fisher, Gibco, catalog number: A2916801)
Hams F12 (Thermo Fisher, Gibco, catalog number: 11765-054)
Rat tail collagen I (made in own lab [12])
Adenine (Sigma-Aldrich, catalog number: A8626)
Hydrochloric acid (HCl) (Sigma-Aldrich, catalog number: H1758)
Dulbecco’s phosphate buffered saline (PBS) (Sigma-Aldrich, catalog number: D5652)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A7979)
Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 655104)
Calcium chloride (CaCl2·2H2O) (Sigma-Aldrich, catalog number: C7902)
Gentamycin sulphate (Sigma-Aldrich, catalog number: G1264)
Cholera enterotoxin (Sigma-Aldrich, catalog number: C8180)
10× minimum essential media (10× MEM) (Thermo Fisher, Gibco, catalog number: 11430-030)
Sodium bicarbonate (Thermo Fisher, Gibco, catalog number: 15750037)
Tissue-Tek® (SAKURA, catalog number: 4583)
Formalin solution neutral buffered, 10% (Sigma-Aldrich, catalog number: HT501128)
Sucrose (Sigma-Aldrich, catalog number: S0389)
Isopentane (Sigma-Aldrich, catalog number: PHR1661)
For proteomics
Lysis buffer, composed of 2% sodium dodecyl sulfate (SDS), 5 mM tris(2-carboxyethyl)phosphine (TCEP), 5.5 mM chloroacetamide (CAA), and 100 mM Tris pH 8.5; used for cell lysis
Acetonitrile (ACN), LC grade (SupelcoTM Analytical, catalog number: 75-05-8), used in various concentrations throughout the protocol, namely 70% for dilution of lysates, 95% for washing proteins, and 40% and 60% for elution on SepPak. ACN is also used for TMT-labeling
Ethanol (anhydrous) (Sigma-Aldrich, catalog number: 459836), used in 70% concentration for washing proteins after aggregation
50 mM ammonium bicarbonate (ABC) (Sigma-Aldrich, catalog number: 09830), used for protein digestion and for resuspending TMT-labeled peptides
50% trifluoroacetic acid (TFA), HPLC (SupelcoTM Analytical, catalog number: TX1276), used for acidification of samples post digestion
1 M TEAB, pH 8 (Thermo ScientificTM, catalog number: 90114), used to achieve a final concentration of 100 mM in TMT-labeling
50% hydroxylamine (Thermo ScientificTM, catalog number: 90115), used to quench the TMT-labeling reaction
0.1% formic acid (v/v) in water (LC–MS grade) (Thermo ScientificTM, catalog number: 85170), used for resuspension of each TMT-labeled sample before pooling
Loading buffer, composed of 80% ACN, 5% TFA, and 1 M glycolic acid (Sigma-Aldrich, catalog number: 8.04104); used for resuspending peptides for phosphopeptide enrichment
1% ammonia (ammonia solution 25%) (Supelco, catalog number: 105428), used for elution of phosphopeptides
5 mM ABC (buffer A) and 100% ACN (buffer B), used in high pH reversed-phase peptide fractionation
PierceTM BCA Protein Assay kit (Thermo ScientificTM, catalog number: 23225)
TMTproTM 16plex label reagent set (Thermo ScientificTM, catalog number: A44520)
LysC, Lysyl endopeptidase (Fujifilm Wako, catalog number: 129-02541)
Trypsin, Sequencing grade modified trypsin (Promega, catalog number: V511X)
Sep-Pak C18 1 cc Vac Cartridge (Waters, catalog number: WAT054955)
Acquity CSH C18 1.7 μm, 1 mm × 150 mm column (Waters, catalog number: 186006935)
Solutions
Adenine (see Recipes)
Cholera enterotoxin (CT) (see Recipes)
Hydrocortisone (HC) (see Recipes)
Epidermal growth factor (EGF) (see Recipes)
Triiodothyronine (T3) (see Recipes)
Calcium chloride (CaCl2) (see Recipes)
Insulin (1,000× for 5 µg/mL) (see Recipes)
Sodium bicarbonate (71.2 mg/mL milli q water) (see Recipes)
Keratinocyte serum free medium (K-SFM) complete (see Recipes)
Organotypic culture medium (see Recipes)
DMEM 10% FBS (DMEM-10) (see Recipes)
Recipes
For organotypic tissue culture
Adenine
Prepare 2.4 mg/mL of adenine in HCl (this is a 100× stock):
Dissolve 243 mg of adenine in 100 mL of 0.05 M HCl.
Stir for approximately 1 h at room temperature (RT).
Filter sterilize with 0.2 μm filters.
Divide into 5 mL aliquots.
Store at -20 °C.
Cholera enterotoxin (CT)
Prepare 10 µM solution of cholera enterotoxin (this is 1,000× stock solution for 100 pM):
Add 1.18 mL of Milli-Q water to a 1 mg vial of cholera enterotoxin to prepare a 10-5 M solution.
Dilute this in 110 mL of PBS.
Filter sterilize with 0.2 µm filters.
Aliquot 2 mL per cryovial.
Store at -20 °C.
Note: MWCT = 84.000 Da
Hydrocortisone (HC)
Prepare 0.2 mg/mL hydrocortisone (this is a 500× stock for 0.4 µg/mL):
Dissolve a 25 mg vial in 5 mL of 100% ethanol. This solution is 5 mg/mL, which is 12.5× for 0.4 µg/mL. This can be stored at -20 °C indefinitely.
Add 2 mL of this concentrated stock to 48 mL of sterile PBS + 0.1% BSA.
Filter-sterilize with 0.2-µm filters.
Aliquot 2 mL into cryovials.
Store at -20 °C.
Epidermal growth factor (EGF)
Prepare 10 µg/mL of EGF (this is a 50,000× stock for 0.2 ng/mL):
Dissolve 100 µg of EGF in 10 mL of PBS + 0.1% BSA.
Filter sterilize with 0.2 µm filters.
Aliquot 1 mL into cryovials.
Store at -20 °C.
Triiodothyronine (T3)
Prepare 20 nM (2 × 10-8 M) of T3 [this is a 1,000× stock for 20 pM (2 × 10-11 M)]:
Measure 6.8 mg of T3 and dissolve in 7.5 mL of 0.02N NaOH.
Add 42.5 mL PBS (this is 100,000× for 20 pM T3).
Store at -20 °C in 10 mL aliquots.
To make the 1000× stock, dilute 0.4 mL of the 100,000× 1:100 into 39.6 mL of PBS.
Filter sterilize with 0.2 µm filters.
Divide into 2 mL aliquots.
Store at -20 °C.
Calcium chloride (CaCl2)
Prepare using calcium dihydrate CaCl2·2H2O (this is a 1,000× stock for 0.3 mM):
Dissolve 4.4 g of CaCl2·2H2O in 100 mL of Milli Q water.
Stir with magnetic bar to dissolve completely.
Filter sterilize with 0.2 µm filters.
Aliquot 2 mL per cryovial.
Store at -20 °C.
Insulin (1,000× for 5 µg/mL)
Use insulin from bovine pancreas.
Dissolve 100 mg of insulin in 20 mL of 0.005 M HCl in a 50 mL blue-capped tube.
Filter sterilize through a celluloseacetate, lowprotein binding 0.2 µM filter.
Aliquot 500 µL per tube.
Store at -20 °C.
Sodium bicarbonate (71.2 mg/mL milliQ water)
For organotypic cultures
Keratinocyte serum free medium (K-SFM) complete
Reagent Final concentration Quantity Keratinocyte-SFM n/a 500 mL CaCl2 (1,000×) 0.3 mM 500 µL BPE (16.8 mg/mL) 25 µg/mL 750 µL EGF (10 µg/mL) 0.2 ng/mL 10 µL Total n/a 501.26 mL Optional: Penicillin/Streptomycin can be added to K-SFM as well (100 U/mL).
Organotypic culture medium
Reagent Final concentration Quantity DMEM n/a 375 mL F12 n/a 125 mL Pen/Strep 100 U/mL 5.14 mL Adenine (2.4 mg/mL stock) 24 µg/mL 5.14 mL HC (0.2 mg/mL stock) 0.4 µg/mL 1 mL CT (10 µM stock) 100 pM 0.514 mL T3 (1,000×) 20 pM 0.514 mL Insulin (1,000×) 5 µg/mL 500 µL FCS 0.3% 1.54 mL Total 514.3 mL DMEM 10% FBS (DMEM-10)
Reagent Final concentration Quantity DMEM n/a 445 mL FBS 10% 50 mL Pen/Strep 100 U/mL 5 mL Total 500 mL
Laboratory supplies
For organotypic tissue culture
Deep-well plates (Corning, catalog number: 355467)
Cell culture insert, PET membrane, 3.0 µm pore size (Corning, catalog number: 353092)
Airlift pads (cut in circles and autoclaved) (Whatman, GE Healthcare, catalog number: 10382461)
Cell culture Petri dishes or flasks from any brand (we use NUNC, Falcon, and Corning)
Equipment
For organotypic tissue culture
CO2 incubator at 37 °C (any brand can be used)
Centrifuge (any brand, for 15 mL tubes and a minimum of 100 g)
Freezers (-20 °C and -80 °C)
Equipment and software for proteomics
KingFisherTM Flex purification system (Thermo Scientific, catalog number: 5400610)
SpeedVac (Eppendor, model: VacufugeTM Concentrator)
Sonicator (Fisherbrand, model: 120 Sonic Dismembrator)
Spectrophotometer (Thermo Scientific, model: NanoDropTM 2000 C)
HPLC system [Thermo Scientific, model: UltiMateTM 3000 Standard (SD)]
Evosep ONE (Evosep)
Mass spectrometer (Thermo Scientific, model: Orbitrap ExplorisTM 480)
Proteome Discoverer software v2.4 (Thermo Scientific)
Chromeleon software v7.2 (Thermo Fisher Scientific)
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Ye, Z., Wandall, H. H. and Dabelsteen, S. (2024). Phosphoproteomic Analysis and Organotypic Cultures for the Study of Signaling Pathways. Bio-protocol 14(4): e4941. DOI: 10.21769/BioProtoc.4941.
- Ye, Z., Kilic, G., Dabelsteen, S., Marinova, I. N., Thøfner, J. F., Song, M., Rudjord-Levann, A. M., Bagdonaite, I., Vakhrushev, S. Y. and Brakebusch, C. H. (2022). Characterization of TGF-β signaling in a human organotypic skin model reveals that loss of TGF-βRII induces invasive tissue growth. Sci. Signal. 15(761): eabo2206. https://www.science.org/doi/10.1126/scisignal.abo2206
分类
细胞生物学 > 细胞分离和培养 > 3D细胞培养
细胞生物学 > 细胞信号传导
分子生物学 > 蛋白质
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link