- EN - English
- CN - 中文
Resolving the In Situ Three-Dimensional Structure of Fly Mechanosensory Organelles Using Serial Section Electron Tomography
利用连续切片电子断层扫描解析果蝇机械感觉细胞器的原位三维结构
(*contributed equally to this work) 发布: 2024年02月20日第14卷第4期 DOI: 10.21769/BioProtoc.4940 浏览次数: 3100
评审: Xiaokang WuRama Reddy GoluguriChen Fan
Abstract
Mechanosensory organelles (MOs) are specialized subcellular entities where force-sensitive channels and supporting structures (e.g., microtubule cytoskeleton) are organized in an orderly manner. The delicate structure of MOs needs to be resolved to understand the mechanisms by which they detect forces and how they are formed. Here, we describe a protocol that allows obtaining detailed information about the nanoscopic ultrastructure of fly MOs by using serial section electron tomography (SS-ET). To preserve fine structural details, the tissues are cryo-immobilized using a high-pressure freezer followed by freeze-substitution at low temperature and embedding in resin at room temperature. Then, sample sections are prepared and used to acquire the dual-axis tilt series images, which are further processed for tomographic reconstruction. Finally, tomograms of consecutive sections are combined into a single larger volume using microtubules as fiducial markers. Using this protocol, we managed to reconstruct the sensory organelles, which provide novel molecular insights as to how fly mechanosensory organelles work and are formed. Based on our experience, we think that, with minimal modifications, this protocol can be adapted to a wide range of applications using different cell and tissue samples.
Key features
• Resolving the high-resolution 3D ultrastructure of subcellular organelles using serial section electron tomography (SS-ET).
• Compared with single-axis tilt series, dual-axis tilt series provides a much wider coverage of Fourier space, improving resolution and features in the reconstructed tomograms.
• The use of high-pressure freezing and freeze-substitution maximally preserves the fine structural details.
Graphical overview
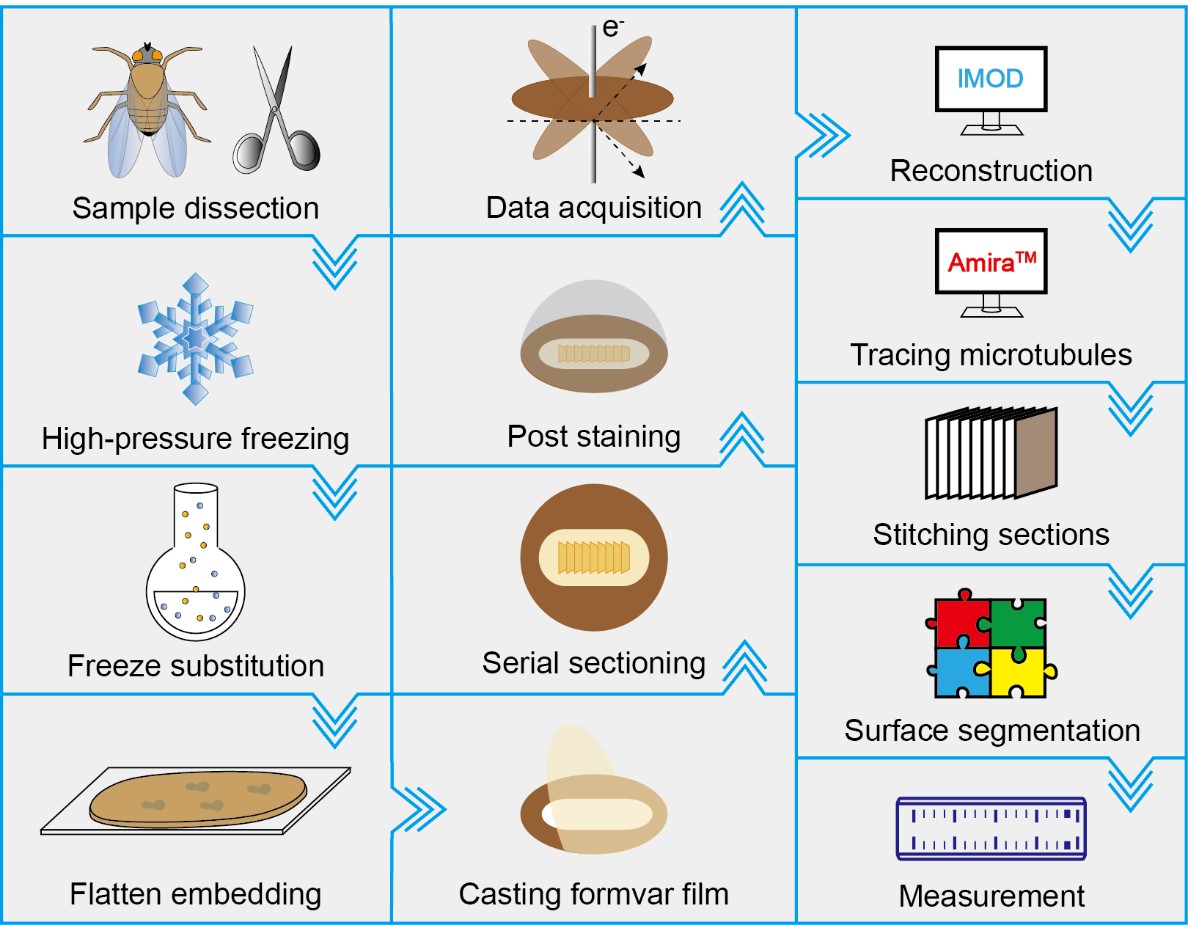
Background
Drosophila type I sensory cells serve as a model for the study of mechanosensation, neuronal cell biology, and ciliogenesis due to their compatibility with functional, imaging, and structural assays [1–3]. In particular, the convenience of performing ultrastructural analysis has facilitated mechanistic studies on the above-mentioned topics, especially for understanding the molecular mechanisms of neurosensory transduction.
Mechanotransduction is the process by which mechanoreceptor cells convert physical signals in the environment into cellular signals [1,2,4]. Mechanoreceptors form a specialized functional entity called mechanosensory organelles (MOs) [5–11]. Because MOs are essentially mechanosensors, they possess a dedicated structural architecture that facilitates the detection of mechanical stimuli. To suit this purpose, MOs develop a delicate intracellular structure [5,7,8,11]. Information regarding this structure is required to understand how mechanosensory cells encode mechanical signals and are formed.
We and others have previously studied the ultrastructure of fly external mechanosensory cells using conventional transmission electron microscopy (TEM)[8,9,12–15]. These studies made a clear presentation of nanoscopic ultrastructures and provided molecular insights. However, the 3D structure of mechanosensory organelles and organization of the sensory molecules are absent. This information allows molecular interpretation of how the force-sensitive molecules transduce mechanical signals and how the specialized structures are formed. To solve these issues, we recently established a protocol for serial section electron tomography (SS-ET) [11,16,17]. Using this protocol, we successfully resolved the delicate structure and molecular organization of the MOs. Together with cell biological and modeling analysis, the structural analysis has been useful in understanding the working mechanism of fly MOs and how they are formed.
Electron tomography (ET) acquires a series of 2D projection images, known as tilt series, by incrementally varying the orientation of the sample relative to the incident electron beam. Using computational methods, the set of 2D images can be processed to yield a tomographic volume reconstruction at nanometer resolution [18,19]. However, the imaging depth of ET is restricted to a few hundred nanometers due to the limited penetration depth of electrons. Therefore, it is recommended that the thickness of plastic samples should be less than 300 nm for the most widely used TEMs. SS-ET, which merges the tomographic volume constructions from consecutive sections, compensates for this limitation to some extent [20,21].
Materials and reagents
Biological materials
Drosophila flies, maintained on a standard medium at 23 to 25 °C
Reagents
Carbon dioxide (CO2) (any supplier)
Sodium dihydrogen phosphate dihydrate (NaH2PO4 ·2H2O) (analytical grade) (e.g., Sinopharm Chemical Reagent Co., Ltd., catalog number: 20040718)
Disodium hydrogen phosphate dodecahydrate (Na2HPO4 ·12H2O) (analytical grade) (e.g., Sinopharm Chemical Reagent Co., Ltd., catalog number: 10020318)
Albumin bovine serum (BSA) (Sigma-Aldrich, catalog number: A9647)
Liquid nitrogen (any supplier)
Osmium tetroxide (Electron Microscopy Sciences, catalog number: 19110)
Glutaraldehyde (25% aqueous solution) (Electron Microscopy Sciences, catalog number: 16220)
Uranyl acetate (Polysciences, catalog number: 21447 or Electron Microscopy Sciences, catalog number: 22400)
Lead citrate (Electron Microscopy Sciences, catalog number: 17800)
Anhydrous acetone (analytical grade) (e.g., Electron Microscopy Sciences, catalog number: 10015)
Sodium hydroxide (NaOH) (Sigma-Aldrich, catalog number: 221465)
Araldite/embed-812 embedding kit (Electron Microscopy Sciences, catalog number: 13940), including embed-812, araldite, DDSA, and DMP-30
Teflon (Miller-Stephenson Chemical Co., Inc., U.S.A.)
Cyanoacrylic adhesive (any supplier)
Chloroform (analytical grade) (e.g., Electron Microscopy Sciences, catalog number: 12540)
Formvar 15/95 resin (Electron Microscopy Sciences, catalog number: 15800)
Methanol (analytical grade) (e.g., RHAWN, catalog number: R007542-4L)
Gold colloid (BBI Solutions, catalog number: EM. GC15)
Solutions
Phosphate buffer (0.1 mol/L, pH 7.2) (see Recipes)
20% BSA solution (see Recipes)
10% osmium tetroxide (see Recipes)
20% uranyl acetate (see Recipes)
Freeze-substitution solution (see Recipes)
30%, 50%, 70% aqueous methanol (see Recipes)
2% uranyl acetate (see Recipes)
10 mol/L NaOH (see Recipes)
0.4% lead citrate (see Recipes)
Araldite/embed-812 embedding media (see Recipes)
1%–3% formvar casting solution (see Recipes)
Recipes
Phosphate buffer (0.1 mol/L, pH 7.2)
Reagent Final concentration Quantity or volume NaH2PO 4·2H2O 0.437% (w/v) 0.437 g Na2HPO4·12H2O 2.579% (w/v) 2.579 g Ultrapure water n/a 100.0 mL Total n/a 100.0 mL 20% BSA (wt/vol) solution
Reagent Final concentration Quantity or volume BSA 20% (w/v) 0.2 g Phosphate buffer (0.1 mol/L, pH 7.2) n/a 0.8 mL Total n/a 1.0 mL 10% osmium tetroxide
Reagent Final concentration Quantity or volume Osmium tetroxide 10% (w/v) 1.0 mg Anhydrous acetone n/a 10 mL Total n/a 10 mL Caution: Osmium tetroxide is highly toxic and fatal. Handle it in a fume hood and wear personal protective equipment.
20% uranyl acetate
Reagent Final concentration Quantity or volume Uranyl acetate 20% (w/v) 0.2 mg Anhydrous methanol n/a 1.0 mL Total n/a 1.0 mL Caution: Uranyl acetate is toxic and slightly radioactive. Handle it in a fume hood and wear personal protective equipment.
Freeze-substitution solution
Reagent Final concentration Quantity or volume 10% osmium tetroxide 1% (w/v) 1.0 mL 20% uranyl acetate 0.1% (w/v) 50 μL 25% aqueous glutaraldehyde 0.5% (w/v) 200 μL Ultrapure water 4% (v/v) 250 μL Anhydrous acetone n/a 8.5 mL Total n/a 10 mL Caution: Freeze-substitution solution contains toxic and radioactive compounds. Handle them inside a fume hood and wear personal protective equipment.
30%, 50%, 70% aqueous methanol
Add 30, 50, and 70 mL of anhydrous methanol into 70, 50, and 30 mL of ultrapure water, respectively. Then, mix each aqueous methanol thoroughly by vortexing. Leave them at room temperature overnight or shake them in an ultrasonic cleaner for ~10 min to remove all air bubbles inside the aqueous methanol.
2% uranyl acetate
Note: Filter the solution using a 0.22 μm syringe filter unit or centrifuge the solution at 10,625× g for 10 min and then collect the filtrate or supernatant to use.
Reagent Final concentration Quantity or volume Uranyl acetate 2% (w/v) 0.2 g 70% aqueous methanol n/a 10 mL Total n/a 10 mL 10 mol/L NaOH
Note: Boil the ultrapure water for 15–30 min and then cool it to room temperature before use.
Reagent Final concentration Quantity or volume NaOH 10 mol/L 4.2 g Cooled boiled water n/a 10 mL Total n/a 10 mL 0.4% lead citrate
Note: Add 0.2 g of lead citrate into 50 mL of cooled boiled water in a 50 mL centrifuge tube. Immediately turn the tubes upside down several times and add 500 μL of 10 mol/L NaOH in it. Shake the tubes on an orbital shaker for at least 30 min and place them in the fume hood for at least two days before use.
Reagent Final concentration Quantity or volume Lead citrate 0.4% (w/v) 0.2 g 10 mol/L NaOH n/a 500 μL Cooled boiled water n/a 50 mL Total n/a 50 mL Araldite/embed-812 embedding media
Reagent Final concentration Quantity or volume Embed-812 n/a 62.0 g Araldite 502 n/a 44.4 g DDSA n/a 122.0 g DMP-30 n/a 5.5 mL Total n/a n/a Caution: Unpolymerized embedding media are toxic. Handle them in a fume hood and wear personal protective equipment.
1%–3% formvar casting solution
Reagent Final concentration Quantity or volume Formvar 15/95 resin 1%–3% (w/v) 1–3 g Chloroform n/a 100 mL Total n/a 100 mL
Laboratory supplies
Microcentrifuge tubes (0.2, 0.5, 1.5, and 2.0 mL) (e.g., Eppendorf, catalog number: 0030124332, 0030121023, 0030120086, and 0030120094, respectively)
Centrifuge tubes (15 and 50 mL) (e.g., Corning, catalog number: 430052 and 430290, respectively)
Petri dishes (30 and 100 mm) (e.g., Corning, catalog number: CLS430165 and CLS430167, respectively)
2 mL screwcap microtubes (Sarstedt AG & Co. KG, catalog number: 72.694.005)
0.22 μm syringe filter unit (Merck Millipore Ltd. catalog number: SLGPR33RS)
Aluminum foil (any supplier)
Pipette tips (0.1–20, 2–200, and 50–1,000 μL) (e.g., Eppendorf, catalog number: 0030000838, 0030000870, and 0030000919, respectively)
Cellulose capillary tubes (Leica Microsystems, catalog number: 16706869)
100 μm deep membrane carriers (Leica Microsystems, catalog number: 16707898)
Stainless steel surgical blades #23 (any supplier)
Surgical blade handles #3 (any supplier)
Plastic beakers (any supplier)
Plastic droppers (any supplier)
Amber glass bottles (any supplier)
Parafilm (Bemis, catalog number: PM996)
Fine tip needle (any supplier)
Glass slides (any supplier)
Slide storage boxes (any supplier)
Single-edge razor blades (any supplier)
Double-edge razor blades (any supplier)
Copper slot grids (Gilder Grids, catalog number: GS2X1-C3)
Filter papers (any supplier)
Cylindrical funnel (any supplier)
ACLAR® 33C film (Electron Microscopy Sciences, catalog number: 50425-10)
Kimwipes tissue (Kimberly-Clark, catalog number: 34155)
Grid storage box (any supplier)
Equipment
Fly anesthesia pad (e.g., Genesee Scientific, catalog number: 59-114)
Tabletop centrifuge (Beckman Coulter, model: Microfuge® 16)
Water purification system for ultrapure water (Merck Millipore, model: Milli-QTM Advantage A10TM)
Superfine vannas scissors (World Precision Instruments, catalog number: 501778)
Sharp forceps (Fine Science Tools, catalog number: 11251-30)
Forceps (Zhongjingkeyi Technology Co. Ltd., catalog number: EZ3-SA)
Pipettes (0.1–2.5, 2–20, 20–200, and 100–1,000 μL) (e.g., Eppendorf, catalog number: 3123000217, 3123000292, 3123000250, and 3123000268, respectively)
Ultrasonic cleaner (e.g., Shenzhen Sweep Technology Co., Ltd., model: SWP-DTP045)
Orbital shaker (e.g., Corning, catalog number: 6780-FP)
Analytical balance (e.g., Mettler-Toledo, catalog number: ME104E)
Stereoscope (Olympus Corporation, catalog number: SZ61)
High-pressure freezer (Leica Microsystems, catalog number: EM HPM100 or EM PACT2)
Automatic freeze-substitution device (Leica Microsystems, catalog number: EM AFS2)
Knifemaker (Leica Microsystems, catalog number: EM KMR3)
Ultramicrotome (Leica Microsystems, catalog number: EM UC7)
35° diamond knife (Diatome, catalog number: DU3530)
Magnetic stirrer (e.g., Heidolph, catalog number: MR3002)
Vortex mixer (e.g., Scientific Industries, model: Vortex-Genie2)
Tube revolver (e.g., Thermo Fisher Scientific, catalog number: 88881002)
Oven (any supplier)
Casting film device (Electron Microscopy Sciences, catalog number: 71305-01)
Grid staining matrix system (Electron Microscopy Sciences, catalog number: 71179-01), including a matrix body with handle and cover, a red staining vessel, and a blue staining vessel
FEI Tecnai F20 TEM equipped with a Gatan US4000 (895) CCD camera and controlled with FEI automated tomography software Xplore 3D
Software and datasets
TEM User Interface software is used in conjunction with TEM Imaging and Analysis controlling software and Digital Micrograph controlling software to acquire micrographs with the TEM
Xplore3D (TEM Tomography Version 3.0) is used for automated tilt series acquisitions
IMOD software package is used to build 3D reconstruction from the raw tilt series [22]
Amira ZIB edition 2016.16 is used for tracing microtubules, stitching consecutive sections, surface segmentation, and measurement
Procedure
文章信息
版权信息
© 2024 The Author(s); This is an open access article under the CC BY-NC license (https://creativecommons.org/licenses/by-nc/4.0/).
如何引用
Readers should cite both the Bio-protocol article and the original research article where this protocol was used:
- Sun, L., Meissner, J., He, J., Cui, L., Fürstenhaupt, T. and Liang, X. (2024). Resolving the In Situ Three-Dimensional Structure of Fly Mechanosensory Organelles Using Serial Section Electron Tomography. Bio-protocol 14(4): e4940. DOI: 10.21769/BioProtoc.4940.
- Song, X., Cui, L., Wu, M., Wang, S., Song, Y., Liu, Z., Xue, Z., Chen, W., Zhang, Y., Li, H., et al. (2023). DCX-EMAP is a core organizer for the ultrastructure of Drosophila mechanosensory organelles. J. Cell Biol. 222(10): e202209116. https://doi.org/10.1083/jcb.202209116
分类
生物物理学 > 电子冷冻断层扫描 > 3D图像重建
细胞生物学 > 细胞结构 > 细胞器
您对这篇实验方法有问题吗?
在此处发布您的问题,我们将邀请本文作者来回答。同时,我们会将您的问题发布到Bio-protocol Exchange,以便寻求社区成员的帮助。
Share
Bluesky
X
Copy link